Riboswitch control of gene expression in plants by splicing and alternative 3' end processing of mRNAs
- PMID: 17993623
- PMCID: PMC2174889
- DOI: 10.1105/tpc.107.053645
Riboswitch control of gene expression in plants by splicing and alternative 3' end processing of mRNAs
Abstract
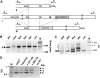
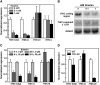
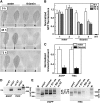
The most widespread riboswitch class, found in organisms from all three domains of life, is responsive to the vitamin B(1) derivative thiamin pyrophosphate (TPP). We have established that a TPP-sensing riboswitch is present in the 3' untranslated region (UTR) of the thiamin biosynthetic gene THIC of all plant species examined. The THIC TPP riboswitch controls the formation of transcripts with alternative 3' UTR lengths, which affect mRNA accumulation and protein production. We demonstrate that riboswitch-mediated regulation of alternative 3' end processing is critical for TPP-dependent feedback control of THIC expression. Our data reveal a mechanism whereby metabolite-dependent alteration of RNA folding controls splicing and alternative 3' end processing of mRNAs. These findings highlight the importance of metabolite sensing by riboswitches in plants and further reveal the significance of alternative 3' end processing as a mechanism of gene control in eukaryotes.
Figures

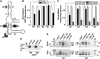


Similar articles
-
Riboswitch-dependent gene regulation and its evolution in the plant kingdom.Genes Dev. 2007 Nov 15;21(22):2874-9. doi: 10.1101/gad.443907. Genes Dev. 2007. PMID: 18006684 Free PMC article.
-
Eukaryotic TPP riboswitch regulation of alternative splicing involving long-distance base pairing.Nucleic Acids Res. 2013 Mar 1;41(5):3022-31. doi: 10.1093/nar/gkt057. Epub 2013 Feb 1. Nucleic Acids Res. 2013. PMID: 23376932 Free PMC article.
-
Analysis of Chlamydomonas thiamin metabolism in vivo reveals riboswitch plasticity.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14622-7. doi: 10.1073/pnas.1307741110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959877 Free PMC article.
-
Riboswitch-mediated control of gene expression in eukaryotes.RNA Biol. 2010 Jan-Feb;7(1):67-76. doi: 10.4161/rna.7.1.10489. Epub 2010 Jan 1. RNA Biol. 2010. PMID: 20009507 Review.
-
Identification of novel ligands for thiamine pyrophosphate (TPP) riboswitches.Biochem Soc Trans. 2011 Apr;39(2):652-7. doi: 10.1042/BST0390652. Biochem Soc Trans. 2011. PMID: 21428956 Review.
Cited by
-
RNA regulatory elements and polyadenylation in plants.Front Plant Sci. 2012 Jan 4;2:109. doi: 10.3389/fpls.2011.00109. eCollection 2011. Front Plant Sci. 2012. PMID: 22629268 Free PMC article.
-
Imaging metabolite dynamics in living cells using a Spinach-based riboswitch.Proc Natl Acad Sci U S A. 2015 May 26;112(21):E2756-65. doi: 10.1073/pnas.1504354112. Epub 2015 May 11. Proc Natl Acad Sci U S A. 2015. PMID: 25964329 Free PMC article.
-
A Theophylline-Responsive Riboswitch Regulates Expression of Nuclear-Encoded Genes.Plant Physiol. 2020 Jan;182(1):123-135. doi: 10.1104/pp.19.00625. Epub 2019 Nov 8. Plant Physiol. 2020. PMID: 31704721 Free PMC article.
-
Cotranscriptional RNA strand exchange underlies the gene regulation mechanism in a purine-sensing transcriptional riboswitch.Nucleic Acids Res. 2022 Nov 28;50(21):12001-12018. doi: 10.1093/nar/gkac102. Nucleic Acids Res. 2022. PMID: 35348734 Free PMC article.
-
Multilayered Regulation of Membrane-Bound ONAC054 Is Essential for Abscisic Acid-Induced Leaf Senescence in Rice.Plant Cell. 2020 Mar;32(3):630-649. doi: 10.1105/tpc.19.00569. Epub 2020 Jan 6. Plant Cell. 2020. PMID: 31911455 Free PMC article.
References
-
- Barrick, J.E., Corbino, K.A., Winkler, W.C., Nahvi, A., Mandal, M., Collins, J., Lee, M., Roth, A., Sudarsan, N., Jona, I., Wickiser, J.K., and Breaker, R.R. (2004). New RNA motifs suggest an expanded scope for riboswitches in bacterial genetic control. Proc. Natl. Acad. Sci. USA 101 6421–6426. - PMC - PubMed
-
- Batey, R.T., Gilbert, S.D., and Montange, R.K. (2004). Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432 411–415. - PubMed
-
- Cazzonelli, C.I., and Velten, J. (2006). An in vivo, luciferase-based, Agrobacterium-infiltration assay system: Implications for post-transcriptional gene silencing. Planta 224 582–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

