Enhancement of protective immune responses by oral vaccination with Saccharomyces cerevisiae expressing recombinant Actinobacillus pleuropneumoniae ApxIA or ApxIIA in mice
- PMID: 17993753
- PMCID: PMC2868155
- DOI: 10.4142/jvs.2007.8.4.383
Enhancement of protective immune responses by oral vaccination with Saccharomyces cerevisiae expressing recombinant Actinobacillus pleuropneumoniae ApxIA or ApxIIA in mice
Abstract
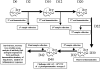
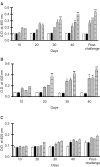
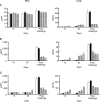
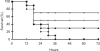
We previously induced protective immune response by oral immunization with yeast expressing the ApxIIA antigen. The ApxI antigen is also an important factor in the protection against Actinobacillus pleuropneumoniae serotype 5 infection; therefore, the protective immunity in mice following oral immunization with Saccharomyces cerevisiae expressing either ApxIA (group C) or ApxIIA (group D) alone or both (group E) was compared with that in two control groups (group A and B). The immunogenicity of the rApxIA antigen derived from the yeast was confirmed by a high survival rate and an ApxIA-specific IgG antibody response (p < 0.01). The highest systemic (IgG) and local (IgA) humoral immune responses to ApxIA and ApxIIA were detected in group E after the third immunization (p < 0.05). The levels of IL-1beta and IL-6 after challenge with an A. pleuropneumoniae field isolate did not change significantly in the vaccinated groups. The level of TNF-alpha increased in a time-dependent manner in group E but was not significantly different after the challenge. After the challenge, the mice in group E had a significantly lower infectious burden and a higher level of protection than the mice in the other groups (p < 0.05). The survival rate in each group was closely correlated to the immune response and histopathological observations in the lung following the challenge. These results suggested that immunity to the ApxIA antigen is required for optimal protection.
Figures



Similar articles
-
Induction of antigen-specific immune responses by oral vaccination with Saccharomyces cerevisiae expressing Actinobacillus pleuropneumoniae ApxIIA.FEMS Immunol Med Microbiol. 2005 Feb 1;43(2):155-64. doi: 10.1016/j.femsim.2004.07.004. FEMS Immunol Med Microbiol. 2005. PMID: 15681145
-
Induction of protective immune responses against challenge of Actinobacillus pleuropneumoniae by oral administration with Saccharomyces cerevisiae expressing Apx toxins in pigs.Vet Immunol Immunopathol. 2013 Jan 15;151(1-2):132-9. doi: 10.1016/j.vetimm.2012.11.003. Epub 2012 Nov 9. Vet Immunol Immunopathol. 2013. PMID: 23206402
-
Immunogenicity and protective efficacy of ApxIA and ApxIIA DNA vaccine against Actinobacillus pleuropneumoniae lethal challenge in murine model.Vaccine. 2009 Jul 23;27(34):4565-70. doi: 10.1016/j.vaccine.2009.05.058. Epub 2009 Jun 9. Vaccine. 2009. PMID: 19520199
-
Oral immunization of mice with Saccharomyces cerevisiae expressing a neutralizing epitope of ApxIIA exotoxin from Actinobacillus pleuropneumoniae induces systemic and mucosal immune responses.Microbiol Immunol. 2013 Jun;57(6):417-25. doi: 10.1111/1348-0421.12053. Microbiol Immunol. 2013. PMID: 23773020
-
New trends in innovative vaccine development against Actinobacillus pleuropneumoniae.Vet Microbiol. 2018 Apr;217:66-75. doi: 10.1016/j.vetmic.2018.02.028. Epub 2018 Mar 6. Vet Microbiol. 2018. PMID: 29615259 Review.
Cited by
-
Oral Administration of Recombinant Saccharomyces boulardii Expressing Ovalbumin-CPE Fusion Protein Induces Antibody Response in Mice.Front Microbiol. 2018 Apr 13;9:723. doi: 10.3389/fmicb.2018.00723. eCollection 2018. Front Microbiol. 2018. PMID: 29706942 Free PMC article.
-
Functional pentameric formation via coexpression of the Escherichia coli heat-labile enterotoxin B subunit and its fusion protein subunit with a neutralizing epitope of ApxIIA exotoxin improves the mucosal immunogenicity and protection against challenge by Actinobacillus pleuropneumoniae.Clin Vaccine Immunol. 2011 Dec;18(12):2168-77. doi: 10.1128/CVI.05230-11. Epub 2011 Oct 26. Clin Vaccine Immunol. 2011. PMID: 22030372 Free PMC article.
-
Recombinant Kluyveromyces lactis expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP5 elicits mucosal and cell-mediated immune responses in mice.J Vet Sci. 2014;15(2):199-208. doi: 10.4142/jvs.2014.15.2.199. Epub 2013 Dec 27. J Vet Sci. 2014. PMID: 24378591 Free PMC article.
-
Plant-derived antigens as mucosal vaccines.Curr Top Microbiol Immunol. 2012;354:101-20. doi: 10.1007/82_2011_158. Curr Top Microbiol Immunol. 2012. PMID: 21811930 Free PMC article. Review.
References
-
- Bathurst IC. Protein expression in yeast as an approach to production of recombinant malaria antigens. Am J Trop Med Hyg. 1994;50:20–26. - PubMed
-
- Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer's patches. Am J Physiol. 1998;275:G130–G137. - PubMed
-
- Blanquet S, Antonelli R, Laforet L, Denis S, Marol-Bonnin S, Alric M. Living recombinant Saccharomyces cerevisiae secreting proteins or peptides as a new drug delivery system in the gut. J Biotechnol. 2004;110:37–49. - PubMed
-
- Boekema BK, Kamp EM, Smits MA, Smith HE, Stockhofe-Zurwieden N. Both ApxI and ApxII of Actinobacillus pleuropneumoniae serotype 1 are necessary for full virulence. Vet Microbiol. 2004;100:17–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

