G protein-coupled receptor sorting to endosomes and lysosomes
- PMID: 17995450
- PMCID: PMC2869288
- DOI: 10.1146/annurev.pharmtox.48.113006.094646
G protein-coupled receptor sorting to endosomes and lysosomes
Abstract
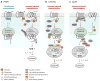
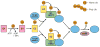
The heptahelical G protein-coupled receptors (GPCRs) belong to the largest family of cell surface signaling receptors encoded in the human genome. GPCRs signal to diverse extracellular stimuli and control a vast number of physiological responses, making this receptor class the target of nearly half the drugs currently in use. In addition to rapid desensitization, receptor trafficking is crucial for the temporal and spatial control of GPCR signaling. Sorting signals present in the intracytosolic domains of GPCRs regulate trafficking through the endosomal-lysosomal system. GPCR internalization is mediated by serine and threonine phosphorylation and arrestin binding. Short, linear peptide sequences including tyrosine- and dileucine-based motifs, and PDZ ligands that are recognized by distinct endocytic adaptor proteins also mediate internalization and endosomal sorting of GPCRs. We present new data from bioinformatic searches that reveal the presence of these types of sorting signals in the cytoplasmic tails of many known GPCRs. Several recent studies also indicate that the covalent modification of GPCRs with ubiquitin serves as a signal for internalization and lysosomal sorting, expanding the diversity of mechanisms that control trafficking of mammalian GPCRs.
Figures
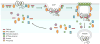
References
-
- Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8:1–9. - PubMed
-
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–55. Excellent comprehensive review on the ubiquitin-dependent endosomal sorting required for transport (ESCRT) machinery. - PubMed
-
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–17. - PubMed
-
- Hirsch JA, Schubert C, Gurevich V, Sigler PA. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–69. - PubMed
-
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, et al. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

