Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1
- PMID: 17995931
- PMCID: PMC4566957
- DOI: 10.1111/j.1471-4159.2007.05039.x
Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S-alkylation of targeted cysteines on Keap1
Abstract
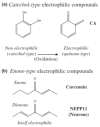
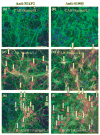
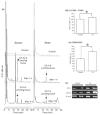
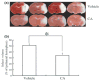
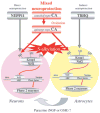
Electrophilic compounds are a newly recognized class of redox-active neuroprotective compounds with electron deficient, electrophilic carbon centers that react with specific cysteine residues on targeted proteins via thiol (S-)alkylation. Although plants produce a variety of physiologically active electrophilic compounds, the detailed mechanism of action of these compounds remains unknown. Catechol ring-containing compounds have attracted attention because they become electrophilic quinones upon oxidation, although they are not themselves electrophilic. In this study, we focused on the neuroprotective effects of one such compound, carnosic acid (CA), found in the herb rosemary obtained from Rosmarinus officinalis. We found that CA activates the Keap1/Nrf2 transcriptional pathway by binding to specific Keap1 cysteine residues, thus protecting neurons from oxidative stress and excitotoxicity. In cerebrocortical cultures, CA-biotin accumulates in non-neuronal cells at low concentrations and in neurons at higher concentrations. We present evidence that both the neuronal and non-neuronal distribution of CA may contribute to its neuroprotective effect. Furthermore, CA translocates into the brain, increases the level of reduced glutathione in vivo, and protects the brain against middle cerebral artery ischemia/reperfusion, suggesting that CA may represent a new type of neuroprotective electrophilic compound.
Figures

Similar articles
-
Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners.Neurosci Lett. 2008 Apr 4;434(3):260-5. doi: 10.1016/j.neulet.2008.01.079. Epub 2008 Feb 13. Neurosci Lett. 2008. PMID: 18329808
-
Simple ortho- and para-hydroquinones as compounds neuroprotective against oxidative stress in a manner associated with specific transcriptional activation.Biochem Biophys Res Commun. 2009 Feb 6;379(2):537-41. doi: 10.1016/j.bbrc.2008.12.106. Epub 2008 Dec 30. Biochem Biophys Res Commun. 2009. PMID: 19118528
-
A novel di terpene para-hydroquinone compound derived from cryptoquinone protects neuronal cells against oxidative stress and activates the Nrf2/ARE pathway.Neurosci Lett. 2013 Aug 26;548:132-6. doi: 10.1016/j.neulet.2013.04.047. Epub 2013 May 3. Neurosci Lett. 2013. PMID: 23648389
-
Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs.Free Radic Biol Med. 2013 Dec;65:645-657. doi: 10.1016/j.freeradbiomed.2013.07.022. Epub 2013 Jul 25. Free Radic Biol Med. 2013. PMID: 23892355 Free PMC article. Review.
-
Recent advances in understanding NRF2 as a druggable target: development of pro-electrophilic and non-covalent NRF2 activators to overcome systemic side effects of electrophilic drugs like dimethyl fumarate.F1000Res. 2017 Dec 14;6:2138. doi: 10.12688/f1000research.12111.1. eCollection 2017. F1000Res. 2017. PMID: 29263788 Free PMC article. Review.
Cited by
-
Callicarpa longissima extract, carnosol-rich, potently inhibits melanogenesis in B16F10 melanoma cells.J Nat Med. 2016 Jan;70(1):28-35. doi: 10.1007/s11418-015-0933-5. Epub 2015 Aug 13. J Nat Med. 2016. PMID: 26267810
-
Redox pioneer: Professor Stuart A. Lipton.Antioxid Redox Signal. 2013 Sep 10;19(8):757-64. doi: 10.1089/ars.2013.5388. Antioxid Redox Signal. 2013. PMID: 23815466 Free PMC article.
-
Carnosic acid mitigates doxorubicin-induced cardiac toxicity: Evidence from animal and cell model investigations.Iran J Basic Med Sci. 2024;27(4):425-438. doi: 10.22038/IJBMS.2023.71508.15544. Iran J Basic Med Sci. 2024. PMID: 38419896 Free PMC article.
-
Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects.Chem Res Toxicol. 2017 Jan 17;30(1):13-37. doi: 10.1021/acs.chemrestox.6b00256. Epub 2016 Sep 29. Chem Res Toxicol. 2017. PMID: 27617882 Free PMC article.
-
Neuroprotective Strategies for Neurological Disorders by Natural Products: An update.Curr Neuropharmacol. 2019;17(3):247-267. doi: 10.2174/1570159X16666180911124605. Curr Neuropharmacol. 2019. PMID: 30207234 Free PMC article. Review.
References
-
- Ahlgren-Beckendorf JA, Reising AM, Schander MA, Herdler JW, Johnson JA. Coordinate regulation of NAD(P)H: quinone oxidoreductase and glutathione-S-transferase in primary cultures of rat neurons and glias: role of the antioxidant/electrophile responsive element. Glia. 1999;15:131–142. - PubMed
-
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a cap’n’collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. - PubMed
-
- Ankacrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
-
- Aruoma OI, Halliwell B, Aeschbach R, Loligers J. Antioxidant and pro oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. - PubMed
-
- Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–1533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

