Oncostatin-M inhibits luteinizing hormone stimulated Leydig cell progenitor formation in vitro
- PMID: 17996055
- PMCID: PMC2174940
- DOI: 10.1186/1477-7827-5-43
Oncostatin-M inhibits luteinizing hormone stimulated Leydig cell progenitor formation in vitro
Abstract
Background: The initial steps of stem Leydig cell differentiation into steroid producing progenitor cells are thought to take place independent of luteinizing hormone (LH), under the influence of locally produced factors such as leukaemia inhibitory factor (LIF), platelet derived growth factor A and stem cell factor. For the formation of a normal sized Leydig cell population in the adult testis, the presence of LH appears to be essential. Oncostatin M (OSM) is a multifunctional cytokine and member of the interleukin (IL)-6 family that also includes other cytokines such as LIF. In the rat OSM is highly expressed in the late fetal and neonatal testis, and may thus be a candidate factor involved in Leydig cell progenitor formation.
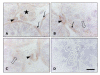
Methods: Interstitial cells were isolated from 13-day-old rat testes and cultured for 1, 3 or 8 days in the presence of different doses of OSM (range: 0.01 to 10 ng/ml) alone or in combination with LH (1 ng/ml). The effects of OSM and LH on cell proliferation were determined by incubating the cultures with [3H]thymidine or bromodeoxyuridine (BrdU). Developing progenitor cells were identified histochemically by the presence of the marker enzyme 3beta-hydroxysteroid dehydrogenase (3beta-HSD).
Results: OSM, when added at a dose of 10 ng/ml, caused a nearly 2-fold increase in the percentage of Leydig cell progenitors after 8 days of culture. Immunohistochemical double labelling experiments with 3beta-HSD and BrdU antibodies showed that this increase was the result of differentiation of stem Leydig cells/precursor cells and not caused by proliferation of progenitor cells themselves. The addition of LH to the cultures consistently resulted in an increase in progenitor formation throughout the culture period. Surprisingly, when OSM and LH were added together, the LH induced rise in progenitor cells was significantly inhibited after 3 and 8 days of culture.
Conclusion: Taken together, the results of the present study suggest that locally produced OSM may not only play a role in the regulation of Sertoli cell proliferation and the initiation of spermatogenesis but may also play a role in the regulation of Leydig cell progenitor formation by keeping the augmenting effects of LH on this process in abeyance.
Figures
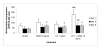
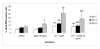

Similar articles
-
Oncostatin M inhibits differentiation of rat stem Leydig cells in vivo and in vitro.J Cell Mol Med. 2019 Jan;23(1):426-438. doi: 10.1111/jcmm.13946. Epub 2018 Oct 15. J Cell Mol Med. 2019. PMID: 30320465 Free PMC article.
-
The development of rat Leydig cell progenitors in vitro: how essential is luteinising hormone?J Endocrinol. 2007 Sep;194(3):579-93. doi: 10.1677/JOE-06-0084. J Endocrinol. 2007. PMID: 17761897
-
Effects of thyroid and luteinizing hormones on the onset of precursor cell differentiation into leydig progenitor cells in the prepubertal rat testis.Biol Reprod. 2000 Sep;63(3):898-904. doi: 10.1095/biolreprod63.3.898. Biol Reprod. 2000. PMID: 10952937
-
Fetal Leydig cell culture--an in vitro system for the study of trophic hormone and GnRH receptors and actions.J Steroid Biochem. 1985 Nov;23(5B):743-55. doi: 10.1016/s0022-4731(85)80010-8. J Steroid Biochem. 1985. PMID: 3001417 Review.
-
Differentiation of the adult Leydig cell population in the postnatal testis.Biol Reprod. 2001 Sep;65(3):660-71. doi: 10.1095/biolreprod65.3.660. Biol Reprod. 2001. PMID: 11514326 Review.
Cited by
-
The clinical relevance of OSM in inflammatory diseases: a comprehensive review.Front Immunol. 2023 Sep 29;14:1239732. doi: 10.3389/fimmu.2023.1239732. eCollection 2023. Front Immunol. 2023. PMID: 37841259 Free PMC article. Review.
-
Oncostatin M inhibits differentiation of rat stem Leydig cells in vivo and in vitro.J Cell Mol Med. 2019 Jan;23(1):426-438. doi: 10.1111/jcmm.13946. Epub 2018 Oct 15. J Cell Mol Med. 2019. PMID: 30320465 Free PMC article.
References
-
- Mendis-Handagama SMLC, Risbridger GP, de Kretser DM. Morphometric analysis of the components of the neonatal and the adult testis interstitium. Int J Androl. 1987;10:525–534. - PubMed
-
- Hardy MP, Zirking BR, Ewing LL. Kinetic studies on the development of the adult population of Leydig cells in the testis of the pubertal rat. Endocrinology. 1989;124:762–770. - PubMed
-
- Haider SG. Cell Biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. - PubMed
-
- Kerr JB, Knell CM. The fate of fetal Leydig cells during the development of the fetal and postnatal testis. Development. 1988;103:535–544. - PubMed
-
- Lording DW, de Kretser DM. Comparative ultrastructural and histochemical studies of the interstitial cells of the rat testis during fetal and postnatal development. J Reprod Fertil. 1972;29:261–269. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

