Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning
- PMID: 17996249
- PMCID: PMC2243222
- DOI: 10.1016/j.jim.2007.09.017
Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning
Erratum in
- J Immunol Methods. 2008 May 20;334(1-2):142
Abstract
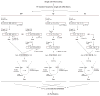
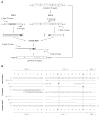
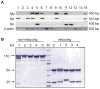
We have developed an efficient strategy that combines immunoglobulin (Ig) gene repertoire analysis and Ig reactivity profiling at the single cell level. Based on surface marker expression individual cells at different stages of human B cell development are isolated by fluorescence-activated cell sorting. For each cell Ig heavy and corresponding Ig light chain gene transcripts are amplified by nested RT-PCR and cloned into eukaryotic expression vectors to produce monoclonal human antibodies of the same specificity in vitro. All reactions are performed in 96-well plates and allow cloning of large numbers of Ig genes. The recombinant antibodies are tested for reactivity with diverse self- and non-self antigens and the reactivity profile can be directly linked to the complete Ig heavy and Ig light chain gene sequence information that is obtained as part of the cloning strategy. In summary, our method to clone and express human monoclonal antibodies is unbiased, highly efficient, requires only small cell numbers and the recombinant antibodies allow direct conclusions on the frequency of specific human B cells in a diverse repertoire.
Figures

References
-
- Brezinschek HP, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995;155:190–202. - PubMed
-
- Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

