The cell biology of Trypanosoma brucei differentiation
- PMID: 17997129
- PMCID: PMC3902322
- DOI: 10.1016/j.mib.2007.09.014
The cell biology of Trypanosoma brucei differentiation
Abstract
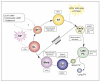
Developmental events in the life-cycle of the sleeping sickness parasite comprise integrated changes in cell morphology, metabolism, gene expression and signalling pathways. In each case these processes differ from the eukaryotic norm. In the past three years, understanding of these developmental processes has progressed from a description of the cytological events of differentiation to a discovery of its underlying molecular controls. With an expanding set of reagents for the identification of distinct parasite life-cycle stages in the tsetse, trypanosome differentiation is being studied from the molecular to the organismal and population level. Interestingly, the new molecular discoveries provide insights into the biology of the parasite in the field.
Figures

References
-
- Aksoy S, Gibson WC, Lehane MJ. Interactions between tsetse and trypanosomes with implications for the control of trypanosomiasis. Adv Parasitol. 2003;53:1–83. - PubMed
-
- Taylor JE, Rudenko G. Switching trypanosome coats: what’s in the wardrobe? Trends Genet. 2006;22:614–620. - PubMed
-
- Robertson M. Notes on the polymorphism of Trypanosoma gambiense in the blood and its relation to the exogenous cycle in Glossina palpalis. Proc Biol Sci. 1912;85:241–539.
-
- Hamm B, Schindler A, Mecke D, Duszenko M. Differentiation of Trypanosoma brucei bloodstream trypomastigotes from long slender to short stumpy-like forms in axenic culture. Mol Biochem Parasitol. 1990;40:13–22. - PubMed
-
- Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

