Bidirectional regulation of Munc13-3 protein expression by age and dark rearing during the critical period in mouse visual cortex
- PMID: 17997229
- PMCID: PMC2189576
- DOI: 10.1016/j.neuroscience.2007.09.053
Bidirectional regulation of Munc13-3 protein expression by age and dark rearing during the critical period in mouse visual cortex
Abstract
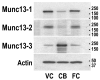
Rearing in darkness slows the time course of the visual cortical critical period, such that at 5 weeks of age normal cats are more plastic than dark-reared cats, while at 20 weeks dark-reared cats are more plastic [Mower GD (1991) The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res 58:151-158]. Thus, genes that are important for visual cortical plasticity should show differences in expression between normal and dark-reared visual cortex that are of opposite direction in young versus older animals. Previously, we showed by differential display polymerase chain reaction and northern blotting that mRNA for Munc13-3, a mammalian homologue of the C. elegans uncoordinated (unc) gene, shows such bidirectional regulation in cat visual cortex [Yang CB, Zheng YT, Li GY, Mower GD (2002) Identification of Munc13-3 as a candidate gene for critical period neuroplasticity in visual cortex. J Neurosci 22:8614-8618]. Here, the analysis is extended to Munc13-3 protein in mouse visual cortex, which will provide the basis for gene manipulation analysis. In mice, Munc13-3 protein was elevated 2.3-fold in dark-reared compared with normal visual cortex at 3.5 weeks and 2.0-fold in normal compared with dark-reared visual cortex at 9.5 weeks. Analysis of variance of protein levels showed a significant interaction, indicating that the effect of dark rearing depended on age. This bidirectional regulation was restricted to visual cortex and did not occur in frontal cortex. Bidirectional regulation was also specific to Munc13-3 and was not found for other Munc13 family members. Munc13 proteins serve a central priming function in synaptic vesicle exocytosis at glutamatergic and GABAergic synapses and this work contributes to the growing evidence indicating a role of Munc13 genes in synaptic plasticity.
Figures


Similar articles
-
Identification of α-chimaerin as a candidate gene for critical period neuronal plasticity in cat and mouse visual cortex.BMC Neurosci. 2011 Jul 18;12:70. doi: 10.1186/1471-2202-12-70. BMC Neurosci. 2011. PMID: 21767388 Free PMC article.
-
Identification of Munc13-3 as a candidate gene for critical-period neuroplasticity in visual cortex.J Neurosci. 2002 Oct 1;22(19):8614-8. doi: 10.1523/JNEUROSCI.22-19-08614.2002. J Neurosci. 2002. PMID: 12351735 Free PMC article.
-
Identification of disabled-1 as a candidate gene for critical period neuroplasticity in cat and mouse visual cortex.Eur J Neurosci. 2006 May;23(10):2804-8. doi: 10.1111/j.1460-9568.2006.04799.x. Eur J Neurosci. 2006. PMID: 16817883
-
Age and dark rearing bidirectionally regulate the level and laminar pattern of expression of Abelson interacting protein 2 (Abi-2): a novel candidate visual cortical plasticity gene.J Mol Neurosci. 2013 Nov;51(3):647-54. doi: 10.1007/s12031-013-0037-1. Epub 2013 Jul 5. J Mol Neurosci. 2013. PMID: 23828391 Free PMC article.
-
Extended plasticity of visual cortex in dark-reared animals may result from prolonged expression of cpg15-like genes.J Neurosci. 2002 Mar 1;22(5):1807-15. doi: 10.1523/JNEUROSCI.22-05-01807.2002. J Neurosci. 2002. PMID: 11880509 Free PMC article.
Cited by
-
Genetics of dementia in a Finnish cohort.Eur J Hum Genet. 2018 Jun;26(6):827-837. doi: 10.1038/s41431-018-0117-3. Epub 2018 Feb 23. Eur J Hum Genet. 2018. PMID: 29476165 Free PMC article.
-
Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis.Mol Biol Cell. 2018 Sep 15;29(19):2259-2279. doi: 10.1091/mbc.E18-04-0225. Epub 2018 Jul 25. Mol Biol Cell. 2018. PMID: 30044717 Free PMC article.
-
TISSUE: uncertainty-calibrated prediction of single-cell spatial transcriptomics improves downstream analyses.Nat Methods. 2024 Mar;21(3):444-454. doi: 10.1038/s41592-024-02184-y. Epub 2024 Feb 12. Nat Methods. 2024. PMID: 38347138
-
Identification of α-chimaerin as a candidate gene for critical period neuronal plasticity in cat and mouse visual cortex.BMC Neurosci. 2011 Jul 18;12:70. doi: 10.1186/1471-2202-12-70. BMC Neurosci. 2011. PMID: 21767388 Free PMC article.
-
Munc13-3 superprimes synaptic vesicles at granule cell-to-basket cell synapses in the mouse cerebellum.J Neurosci. 2014 Oct 29;34(44):14687-96. doi: 10.1523/JNEUROSCI.2060-14.2014. J Neurosci. 2014. PMID: 25355221 Free PMC article.
References
-
- Abel T, Martin KC, Bartsch D, Kandel ER. Memory suppressor genes: inhibitory constraints on the storage of long-term memory. Science. 1998;279:338–341. - PubMed
-
- Artola A, Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987;330:649–652. - PubMed
-
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999b;400:457–461. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

