How sugars convey information on protein conformation in the endoplasmic reticulum
- PMID: 17997334
- PMCID: PMC2196135
- DOI: 10.1016/j.semcdb.2007.09.006
How sugars convey information on protein conformation in the endoplasmic reticulum
Abstract
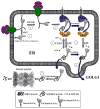
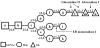
The N-glycan-dependent quality control of glycoprotein folding prevents endoplasmic reticulum to Golgi exit of folding intermediates, irreparably misfolded glycoproteins and not completely assembled multimeric complexes. It also enhances folding efficiency by preventing aggregation and facilitating formation of proper disulfide bonds. The control mechanism essentially involves four components, resident lectin-chaperones that recognize monoglucosylated polymannose glycans, a lectin-associated oxidoreductase acting on monoglucosylated glycoproteins, a glucosyltransferase and a glucosidase that creates monoglucosylated epitopes in glycans transferred in protein N-glycosylation or removes the glucose units added by the glucosyltransferase. This last enzyme is the only mechanism component sensing glycoprotein conformations as it creates monoglucosylated glycans exclusively in not properly folded species or in not completely assembled complexes. The purpose of the review is to describe the most significant recent findings on the mechanism of glycoprotein folding and assembly quality control and to discuss the main still unanswered questions.
Figures
References
-
- Alonso JM, Santa-Cecilia A, Calvo P. Effect of bromoconduritol on glucosidase II from rat liver. A new kinetic model for the binding and hydrolysis of the substrate. Eur J Biochem. 1993;215:37–42. - PubMed
-
- Arnold SM, Kaufman RJ. The noncatalytic portion of human UDP-glucose:glycoprotein glucosyltransferase I confers UDP-glucose binding and transferase function to the catalytic domain. J Biol Chem. 2003;278:43320–8. - PubMed
-
- Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem. 1991;266:21458–65. - PubMed
-
- Bouvier M, Stafford WF. Probing the three-dimensional structure of human calreticulin. Biochemistry. 2000;39:14950–59. - PubMed
-
- Cannon KS, Hebert DN, Helenius A. Glycan-dependent and -independent association of vesicular stomatitis virus G protein with calnexin. J Biol Chem. 1996;271:14280–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

