Increased catalytic efficiency following gene fusion of bifunctional methionine sulfoxide reductase enzymes from Shewanella oneidensis
- PMID: 17997579
- PMCID: PMC2546871
- DOI: 10.1021/bi701151t
Increased catalytic efficiency following gene fusion of bifunctional methionine sulfoxide reductase enzymes from Shewanella oneidensis
Abstract
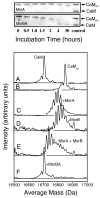
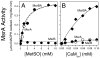
Methionine sulfoxide reductase enzymes MsrA and MsrB have complementary stereospecificities that reduce the S and R stereoisomers of methionine sulfoxide (MetSO), respectively, and together function as critical antioxidant enzymes. In some pathogenic and metal-reducing bacteria, these genes are fused to form a bifunctional methionine sulfoxide reductase (i.e., MsrBA) enzyme. To investigate how gene fusion affects the substrate specificity and catalytic activities of Msr, we have cloned and expressed the MsrBA enzyme from Shewanella oneidensis, a metal-reducing bacterium and fish pathogen. For comparison, we also cloned and expressed the wild-type MsrA enzyme from S. oneidensis and a genetically engineered MsrB protein. MsrBA is able to completely reduce (i.e., repair) MetSO in the calcium regulatory protein calmodulin (CaM), while only partial repair is observed using both MsrA and MsrB enzymes together at 25 degrees C. A restoration of the normal protein fold is observed co-incident with the repair of MetSO in oxidized CaM (CaMox by MsrBA, as monitored by time-dependent increases in the anisotropy associated with the rigidly bound multiuse affinity probe 4',5'-bis(1,3,2-dithioarsolan-2-yl)fluorescein (FlAsH). Underlying the efficient repair of MetSO in CaMox is the coordinate activity of the two catalytic domains in the MsrBA fusion protein, which results in a 1 order of magnitude rate enhancement in comparison to those of the individual MsrA or MsrB enzyme alone. The coordinate binding of both domains of MsrBA permits the full repair of all MetSO in CaMox. The common expression of Msr fusion proteins in bacterial pathogens is consistent with an important role for this enzyme activity in the maintenance of protein function necessary for bacterial survival under highly oxidizing conditions associated with pathogenesis or bioremediation.
Figures




Similar articles
-
Repair of oxidized calmodulin by methionine sulfoxide reductase restores ability to activate the plasma membrane Ca-ATPase.Biochemistry. 1999 Jan 5;38(1):105-12. doi: 10.1021/bi981295k. Biochemistry. 1999. PMID: 9890888
-
The Arabidopsis plastidic methionine sulfoxide reductase B proteins. Sequence and activity characteristics, comparison of the expression with plastidic methionine sulfoxide reductase A, and induction by photooxidative stress.Plant Physiol. 2005 Jun;138(2):909-22. doi: 10.1104/pp.105.062430. Epub 2005 May 27. Plant Physiol. 2005. PMID: 15923321 Free PMC article.
-
High-affinity and cooperative binding of oxidized calmodulin by methionine sulfoxide reductase.Biochemistry. 2006 Dec 12;45(49):14642-54. doi: 10.1021/bi0612465. Biochemistry. 2006. PMID: 17144657
-
The methionine sulfoxide reductases: Catalysis and substrate specificities.Arch Biochem Biophys. 2008 Jun 15;474(2):266-73. doi: 10.1016/j.abb.2008.02.007. Epub 2008 Feb 13. Arch Biochem Biophys. 2008. PMID: 18302927 Review.
-
Methionine sulfoxide reductases and virulence of bacterial pathogens.Future Microbiol. 2007 Dec;2(6):619-30. doi: 10.2217/17460913.2.6.619. Future Microbiol. 2007. PMID: 18041903 Review.
Cited by
-
Structural Insights into a Bifunctional Peptide Methionine Sulfoxide Reductase MsrA/B Fusion Protein from Helicobacter pylori.Antioxidants (Basel). 2021 Mar 5;10(3):389. doi: 10.3390/antiox10030389. Antioxidants (Basel). 2021. PMID: 33807684 Free PMC article.
-
Methionine Sulfoxide Reductases of Archaea.Antioxidants (Basel). 2018 Sep 20;7(10):124. doi: 10.3390/antiox7100124. Antioxidants (Basel). 2018. PMID: 30241308 Free PMC article. Review.
-
The urea carboxylase and allophanate hydrolase activities of urea amidolyase are functionally independent.Protein Sci. 2016 Oct;25(10):1812-24. doi: 10.1002/pro.2990. Epub 2016 Aug 5. Protein Sci. 2016. PMID: 27452902 Free PMC article.
-
Comprehensive Temporal Protein Dynamics during Postirradiation Recovery in Deinococcus radiodurans.Oxid Med Cell Longev. 2022 Nov 11;2022:1622829. doi: 10.1155/2022/1622829. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36411759 Free PMC article.
-
Identification of methionine sulfoxide diastereomers in immunoglobulin gamma antibodies using methionine sulfoxide reductase enzymes.MAbs. 2010 May-Jun;2(3):299-308. doi: 10.4161/mabs.2.3.11755. Epub 2010 May 11. MAbs. 2010. PMID: 20404551 Free PMC article.
References
-
- Stadtman ER, Van Remmen H, Richardson A, Wehr NB, Levine RL. Methionine oxidation and aging. Biochim Biophys Acta. 2005;1703:135–140. - PubMed
-
- Schoneich C. Methionine oxidation by reactive oxygen species: reaction mechanisms and relevance to Alzheimer’s disease. Biochim Biophys Acta. 2005;1703:111–119. - PubMed
-
- Boschi-Muller S, Olry A, Antoine M, Branlant G. The enzymology and biochemistry of methionine sulfoxide reductases. Biochimica et Biophysica Acta (BBA) -Proteins & Proteomics. 2005;1703:231–238. - PubMed
-
- Xiong Y, Chen B, Smallwood HS, Urbauer RJ, Markille LM, Galeva N, Williams TD, Squier TC. High-affinity and cooperative binding of oxidized calmodulin by methionine sulfoxide reductase. Biochemistry. 2006;45:14642–14654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

