Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature
- PMID: 17997605
- PMCID: PMC2065874
- DOI: 10.1371/journal.ppat.0030171
Release of hepatic Plasmodium yoelii merozoites into the pulmonary microvasculature
Abstract
Plasmodium undergoes one round of multiplication in the liver prior to invading erythrocytes and initiating the symptomatic blood phase of the malaria infection. Productive hepatocyte infection by sporozoites leads to the generation of thousands of merozoites capable of erythrocyte invasion. Merozoites are released from infected hepatocytes as merosomes, packets of hundreds of parasites surrounded by host cell membrane. Intravital microscopy of green fluorescent protein-expressing P. yoelii parasites showed that the majority of merosomes exit the liver intact, adapt a relatively uniform size of 12-18 microm, and contain 100-200 merozoites. Merosomes survived the subsequent passage through the right heart undamaged and accumulated in the lungs. Merosomes were absent from blood harvested from the left ventricle and from tail vein blood, indicating that the lungs effectively cleared the blood from all large parasite aggregates. Accordingly, merosomes were not detectable in major organs such as brain, kidney, and spleen. The failure of annexin V to label merosomes collected from hepatic effluent indicates that phosphatidylserine is not exposed on the surface of the merosome membrane suggesting the infected hepatocyte did not undergo apoptosis prior to merosome release. Merosomal merozoites continued to express green fluorescent protein and did not incorporate propidium iodide or YO-PRO-1 indicating parasite viability and an intact merosome membrane. Evidence of merosomal merozoite infectivity was provided by hepatic effluent containing merosomes being significantly more infective than blood with an identical low-level parasitemia. Ex vivo analysis showed that merosomes eventually disintegrate inside pulmonary capillaries, thus liberating merozoites into the bloodstream. We conclude that merosome packaging protects hepatic merozoites from phagocytic attack by sinusoidal Kupffer cells, and that release into the lung microvasculature enhances the chance of successful erythrocyte invasion. We believe this previously unknown part of the plasmodial life cycle ensures an effective transition from the liver to the blood phase of the malaria infection.
Conflict of interest statement
Figures

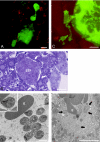




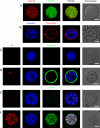
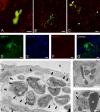
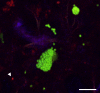
Similar articles
-
Overexpression of Plasmodium berghei ATG8 by Liver Forms Leads to Cumulative Defects in Organelle Dynamics and to Generation of Noninfectious Merozoites.mBio. 2016 Jun 28;7(3):e00682-16. doi: 10.1128/mBio.00682-16. mBio. 2016. PMID: 27353755 Free PMC article.
-
Intravital microscopy of the spleen: quantitative analysis of parasite mobility and blood flow.J Vis Exp. 2012 Jan 14;(59):3609. doi: 10.3791/3609. J Vis Exp. 2012. PMID: 22298018 Free PMC article.
-
Moving on: How malaria parasites exit the liver.Mol Microbiol. 2024 Mar;121(3):328-340. doi: 10.1111/mmi.15141. Epub 2023 Aug 21. Mol Microbiol. 2024. PMID: 37602900 Review.
-
Hostile takeover by Plasmodium: reorganization of parasite and host cell membranes during liver stage egress.PLoS Pathog. 2011 Sep;7(9):e1002224. doi: 10.1371/journal.ppat.1002224. Epub 2011 Sep 1. PLoS Pathog. 2011. PMID: 21909271 Free PMC article.
-
Same, same but different: Exploring Plasmodium cell division during liver stage development.PLoS Pathog. 2023 Mar 30;19(3):e1011210. doi: 10.1371/journal.ppat.1011210. eCollection 2023 Mar. PLoS Pathog. 2023. PMID: 36996035 Free PMC article. Review.
Cited by
-
Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice.Nat Commun. 2015 Jul 24;6:7690. doi: 10.1038/ncomms8690. Nat Commun. 2015. PMID: 26205537 Free PMC article.
-
Clinical trial in healthy malaria-naïve adults to evaluate the safety, tolerability, immunogenicity and efficacy of MuStDO5, a five-gene, sporozoite/hepatic stage Plasmodium falciparum DNA vaccine combined with escalating dose human GM-CSF DNA.Hum Vaccin Immunother. 2012 Nov 1;8(11):1564-84. doi: 10.4161/hv.22129. Epub 2012 Nov 1. Hum Vaccin Immunother. 2012. PMID: 23151451 Free PMC article. Clinical Trial.
-
Exoerythrocytic Plasmodium parasites secrete a cysteine protease inhibitor involved in sporozoite invasion and capable of blocking cell death of host hepatocytes.PLoS Pathog. 2010 Mar 26;6(3):e1000825. doi: 10.1371/journal.ppat.1000825. PLoS Pathog. 2010. PMID: 20361051 Free PMC article.
-
Forward Genetics in Apicomplexa Biology: The Host Side of the Story.Front Cell Infect Microbiol. 2022 May 12;12:878475. doi: 10.3389/fcimb.2022.878475. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646724 Free PMC article. Review.
-
Malaria Elimination in Africa: Rethinking Strategies for Plasmodium vivax and Lessons from Botswana.Trop Med Infect Dis. 2023 Jul 31;8(8):392. doi: 10.3390/tropicalmed8080392. Trop Med Infect Dis. 2023. PMID: 37624330 Free PMC article. Review.
References
-
- Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei . Nature. 1967;216:160–162. - PubMed
-
- Epstein JE, Giersing B, Mullen G, Moorthy V, Richie TL. Malaria vaccines: are we getting closer? Curr Opin Mol Ther. 2007;9:12–24. - PubMed
-
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilization of Plasmodium berghei sporozoites injected into skin by mosquitoes. Int J Parasitol. 2004;34:991–996. - PubMed
-
- Amino R, Thiberge S, Martin B, Celli S, Shorte S, et al. Quantitative imaging of Plasmodium transmission from mosquito to mammal. Nat Med. 2006;12:220–224. - PubMed
-
- Meis JFGM, Verhave JP. Exoerythrocytic development of malaria parasites. Adv Parasitol. 1988;27:1–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

