Niacin restriction upregulates NADPH oxidase and reactive oxygen species (ROS) in human keratinocytes
- PMID: 17997992
- PMCID: PMC2323356
- DOI: 10.1016/j.freeradbiomed.2007.10.006
Niacin restriction upregulates NADPH oxidase and reactive oxygen species (ROS) in human keratinocytes
Abstract
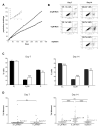
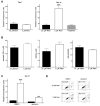
NAD(+) is a substrate for many enzymes, including poly(ADP-ribose) polymerases and sirtuins, which are involved in fundamental cellular processes including DNA repair, stress responses, signaling, transcription, apoptosis, metabolism, differentiation, chromatin structure, and life span. Because these molecular processes are important early in cancer development, we developed a model to identify critical NAD-dependent pathways potentially important in early skin carcinogenesis. Removal of niacin from the cell culture medium allowed control of intracellular NAD. Unlike many nonimmortalized human cells, HaCaT keratinocytes, which are immortalized and have a mutant p53 and aberrant NF-kB activity, become severely NAD depleted but divide indefinitely under these conditions. Niacin-deficient HaCaTs develop a decreased growth rate due to an increase in apoptotic cells and an arrest in the G(2)/M phase of the cell cycle. Long-term survival mechanisms in niacin-deficient HaCats involve accumulation of reactive oxygen species and increased DNA damage. These alterations result, at least in part, from increased expression and activity of NADPH oxidase, whose downstream effects can be reversed by nicotinamide or NADPH oxidase inhibitors. Our data support the hypothesis that glutamine is a likely alternative energy source during niacin deficiency and we suggest a model for NADPH generation important in ROS production.
Figures


References
-
- Ziegler M. New functions of a long-known molecule. Emerging roles of NAD in cellular signaling. Eur J Biochem. 2000;267:1550–1564. - PubMed
-
- Jacobson MK, Jacobson EL. Discovering new ADP-ribose polymer cycles: protecting the genome and more. Trends Biochem Sci. 1999;24:415–417. - PubMed
-
- Durkacz BW, Omidiji O, Gray DA, Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980;283:593–596. - PubMed
-
- Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356:356–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

