Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation
- PMID: 17998387
- PMCID: PMC2118532
- DOI: 10.1084/jem.20070366
Phosphoinositide 3-kinase gamma participates in T cell receptor-induced T cell activation
Abstract
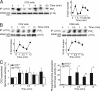
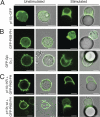
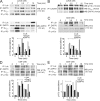
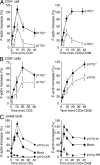
Class I phosphoinositide 3-kinases (PI3Ks) constitute a family of enzymes that generates 3-phosphorylated polyphosphoinositides at the cell membrane after stimulation of protein tyrosine (Tyr) kinase-associated receptors or G protein-coupled receptors (GPCRs). The class I PI3Ks are divided into two types: class I(A) p85/p110 heterodimers, which are activated by Tyr kinases, and the class I(B) p110gamma isoform, which is activated by GPCR. Although the T cell receptor (TCR) is a protein Tyr kinase-associated receptor, p110gamma deletion affects TCR-induced T cell stimulation. We examined whether the TCR activates p110gamma, as well as the consequences of interfering with p110gamma expression or function for T cell activation. We found that after TCR ligation, p110gamma interacts with G alpha(q/11), lymphocyte-specific Tyr kinase, and zeta-associated protein. TCR stimulation activates p110gamma, which affects 3-phosphorylated polyphosphoinositide levels at the immunological synapse. We show that TCR-stimulated p110gamma controls RAS-related C3 botulinum substrate 1 activity, F-actin polarization, and the interaction between T cells and antigen-presenting cells, illustrating a crucial role for p110gamma in TCR-induced T cell activation.
Figures



References
-
- Fruman, D.A. 2004. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr. Opin. Immunol. 16:314–320. - PubMed
-
- Okkenhaug, K., and B. Vanhaesebroeck. 2003. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 3:317–330. - PubMed
-
- Stephens, L.R., A. Eguinoa, H. Erdjument-Bromage, M. Lui, F. Cooke, J. Coadwell, A.S. Smrcka, M. Thelen, K. Cadwallader, P. Tempst, and P.T. Hawkins. 1997. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 89:105–114. - PubMed
-
- Lopez-Ilasaca, M., P. Crespo, P.G. Pellici, J.S. Gutkind, and R. Wetzker. 1997. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 275:394–397. - PubMed
-
- Stoyanov, B., S. Volinia, T. Hanck, I. Rubio, M. Loubtchenkov, D. Malek, S. Stoyanova, B. Vanhaesebroeck, R. Dhand, B. Nürnberg, et al. 1995. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 269:690–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

