A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses
- PMID: 17998391
- PMCID: PMC2118509
- DOI: 10.1084/jem.20071132
A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses
Abstract
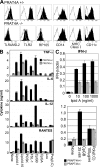
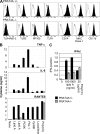
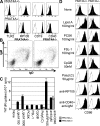
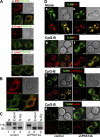
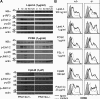
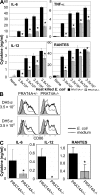
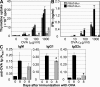
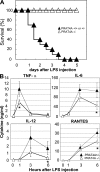
Immune cells express multiple Toll-like receptors (TLRs) that are concomitantly activated by a variety of pathogen products. Although there is presumably a need to coordinate the expression and function of TLRs in individual cells, little is known about the mechanisms governing this process. We show that a protein associated with TLR4 (PRAT4A) is required for multiple TLR responses. PRAT4A resides in the endoplasmic reticulum, and PRAT4A knockdown inhibited trafficking of TLR1 and TLR4 to the cell surface and ligand-induced trafficking of TLR9 to lysosomes. Other cell-surface molecules were expressed normally on immunocytes from PRAT4A-/- mice. There was impaired cytokine production to TLR ligands, except to the TLR3 ligand poly(I:C), and to whole bacteria. Activation of antigen-specific T helper type 1 responses were also defective. Moreover, PRAT4A-/- bone marrow chimeric mice were resistant to lipopolysaccharide-induced sepsis. These results suggest that PRAT4A regulates the subcellular distribution and response of multiple TLRs and is required for both innate and adaptive immune responses.
Figures
References
-
- Beutler, B., Z. Jiang, P. Georgel, K. Crozat, B. Croker, S. Rutschmann, X. Du, and K. Hoebe. 2006. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24:353–389. - PubMed
-
- Kaisho, T., and S. Akira. 2006. Toll-like receptor function and signaling. J. Allergy Clin. Immunol. 117:979–987. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

