A segment of gamma ENaC mediates elastase activation of Na+ transport
- PMID: 17998393
- PMCID: PMC2151661
- DOI: 10.1085/jgp.200709781
A segment of gamma ENaC mediates elastase activation of Na+ transport
Abstract
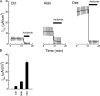
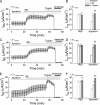
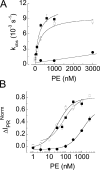
The epithelial Na(+) channel (ENaC) that mediates regulated Na(+) reabsorption by epithelial cells in the kidney and lungs can be activated by endogenous proteases such as channel activating protease 1 and exogenous proteases such as trypsin and neutrophil elastase (NE). The mechanism by which exogenous proteases activate the channel is unknown. To test the hypothesis that residues on ENaC mediate protease-dependent channel activation wild-type and mutant ENaC were stably expressed in the FRT epithelial cell line using a tripromoter human ENaC construct, and protease-induced short-circuit current activation was measured in aprotinin-treated cells. The amiloride-sensitive short circuit current (I(Na)) was stimulated by aldosterone (1.5-fold) and dexamethasone (8-fold). Dexamethasone-treated cells were used for all subsequent studies. The serum protease inhibitor aprotinin decreased baseline I(Na) by approximately 50% and I(Na) could be restored to baseline control values by the exogenous addition of trypsin, NE, and porcine pancreatic elastase (PE) but not by thrombin. All protease experiments were thus performed after exposure to aprotinin. Because NE recognition of substrates occurs with a preference for binding valines at the active site, several valines in the extracellular loops of alpha and gamma ENaC were sequentially substituted with glycines. This scan yielded two valine residues in gamma ENaC at positions 182 and 193 that resulted in inhibited responses to NE when simultaneously changed to other amino acids. The mutations resulted in decreased rates of activation and decreased activated steady-state current levels. There was an approximately 20-fold difference in activation efficiency of NE against wild-type ENaC compared to a mutant with glycine substitutions at positions 182 and 193. However, the mutants remain susceptible to activation by trypsin and the related elastase, PE. Alanine is the preferred P(1) position residue for PE and substitution of alanine 190 in the gamma subunit eliminated I(Na) activation by PE. Further, substitution with a novel thrombin consensus sequence (LVPRG) beginning at residue 186 in the gamma subunit (gamma(Th)) allowed for I(Na) activation by thrombin, whereas wild-type ENaC was unresponsive. MALDI-TOF mass spectrometric evaluation of proteolytic digests of a 23-mer peptide encompassing the identified residues (T(176)-S(198)) showed that hydrolysis occurred between residues V193 and M194 for NE and between A190 and S191 for PE. In vitro translation studies demonstrated thrombin cleaved the gamma(Th) but not the wild-type gamma subunit. These results demonstrate that gamma subunit valines 182 and 193 are critical for channel activation by NE, alanine 190 is critical for channel activation by PE, and that channel activation can be achieved by inserting a novel thrombin consensus sequence. These results support the conclusion that protease binding and perhaps cleavage of the gamma subunit results in ENaC activation.
Figures






References
-
- Andreasen, D., G. Vuagniaux, N. Fowler-Jaeger, E. Hummler, and B.C. Rossier. 2006. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J. Am. Soc. Nephrol. 17:968–976. - PubMed
-
- Auerswald, E.A., D. Horlein, G. Reinhardt, W. Schroder, and E. Schnabel. 1988. Expression, isolation and characterization of recombinant [Arg15,Glu52]aprotinin. Biol. Chem. Hoppe Seyler. 369(Suppl.):27–35. - PubMed
-
- Baugh, R.J., and J. Travis. 1976. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 15:836–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

