Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo
- PMID: 17998900
- PMCID: PMC4376281
- DOI: 10.1038/sj.mt.6300348
Myxoma virus oncolysis of primary and metastatic B16F10 mouse tumors in vivo
Abstract
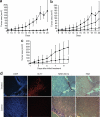
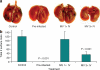
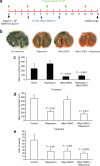
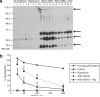
Myxoma virus (MV) is a rabbit-specific poxvirus, whose unexpected tropism to human cancer cells has led to studies exploring its potential use in oncolytic therapy. MV infects a wide range of human cancer cells in vitro, in a manner intricately linked to the cellular activation of Akt kinase. MV has also been successfully used for treating human glioma xenografts in immunodeficient mice. This study examines the effectiveness of MV in treating primary and metastatic mouse tumors in immunocompetent C57BL6 mice. We have found that several mouse tumor cell lines, including B16 melanomas, are permissive to MV infection. B16F10 cells were used for assessing MV replication and efficacy in syngeneic primary tumor and metastatic models in vivo. Multiple intratumoral injections of MV resulted in dramatic inhibition of tumor growth. Systemic administration of MV in a lung metastasis model with B16F10LacZ cells was dramatically effective in reducing lung tumor burden. Combination therapy of MV with rapamycin reduced both size and number of lung metastases, and also reduced the induced antiviral neutralizing antibody titres, but did not affect tumor tropism. These results show MV to be a promising virotherapeutic agent in immunocompetent animal tumor models, with good efficacy in combination with rapamycin.
Figures


Similar articles
-
Targeting human medulloblastoma: oncolytic virotherapy with myxoma virus is enhanced by rapamycin.Cancer Res. 2007 Sep 15;67(18):8818-27. doi: 10.1158/0008-5472.CAN-07-1214. Cancer Res. 2007. PMID: 17875723 Free PMC article.
-
Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors.Cancer Immunol Immunother. 2011 Oct;60(10):1461-72. doi: 10.1007/s00262-011-1045-z. Epub 2011 Jun 9. Cancer Immunol Immunother. 2011. PMID: 21656158 Free PMC article.
-
Oncolytic virotherapy synergism with signaling inhibitors: Rapamycin increases myxoma virus tropism for human tumor cells.J Virol. 2007 Feb;81(3):1251-60. doi: 10.1128/JVI.01408-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108021 Free PMC article.
-
The role of cell signaling in poxvirus tropism: the case of the M-T5 host range protein of myxoma virus.Biochim Biophys Acta. 2008 Jan;1784(1):228-37. doi: 10.1016/j.bbapap.2007.08.001. Epub 2007 Aug 14. Biochim Biophys Acta. 2008. PMID: 17905673 Review.
-
Myxoma virus and oncolytic virotherapy: a new biologic weapon in the war against cancer.Expert Opin Biol Ther. 2007 Sep;7(9):1415-25. doi: 10.1517/14712598.7.9.1415. Expert Opin Biol Ther. 2007. PMID: 17727330 Review.
Cited by
-
Oncolytic myxoma virus: the path to clinic.Vaccine. 2013 Sep 6;31(39):4252-8. doi: 10.1016/j.vaccine.2013.05.056. Epub 2013 May 29. Vaccine. 2013. PMID: 23726825 Free PMC article. Review.
-
Oncolytic Myxoma virus Increases Autophagy in Multiple Myeloma.Turk J Haematol. 2024 Mar 1;41(1):16-25. doi: 10.4274/tjh.galenos.2024.2023.0403. Epub 2024 Jan 23. Turk J Haematol. 2024. PMID: 38258554 Free PMC article.
-
ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic.Mol Ther. 2012 Jun;20(6):1148-57. doi: 10.1038/mt.2011.301. Epub 2012 Jan 24. Mol Ther. 2012. PMID: 22273579 Free PMC article.
-
Histological evaluation of intratumoral myxoma virus treatment in an immunocompetent mouse model of melanoma.Oncolytic Virother. 2013 Jan;2:1-17. doi: 10.2147/OV.S37971. Oncolytic Virother. 2013. PMID: 25866742 Free PMC article.
-
Treatment of an Alveolar Rhabdomyosarcoma Allograft with Recombinant Myxoma Virus and Oclacitinib.Oncolytic Virother. 2020 May 26;9:17-29. doi: 10.2147/OV.S252727. eCollection 2020. Oncolytic Virother. 2020. PMID: 32548076 Free PMC article.
References
-
- Dock G. Influence of complicating diseases upon leukemia. Am J Med Sci. 1904;127:563–592.
-
- Bell JC, Garson KA, Lichty BD, Stojdl DF. Oncolytic viruses: programmable tumour hunters. Curr Gene Ther. 2002;2:243–254. - PubMed
-
- Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7:106–119. - PubMed
-
- Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. - PubMed
-
- Yu DC, Working P, Ando D. Selectively replicating oncolytic adenoviruses as cancer therapeutics. Curr Opin Mol Ther. 2002;4:435–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

