Proton-coupled electron transfer
- PMID: 17999556
- PMCID: PMC3449329
- DOI: 10.1021/cr0500030
Proton-coupled electron transfer
Figures
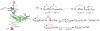
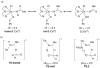
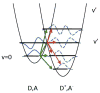
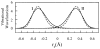
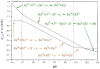

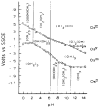
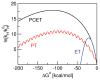

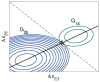






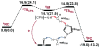
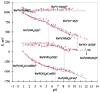
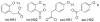
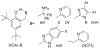
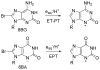
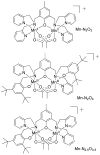
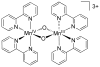
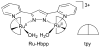
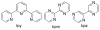
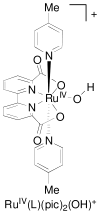
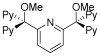
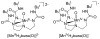

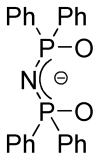
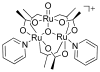
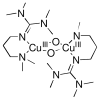

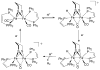


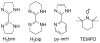
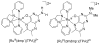
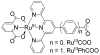
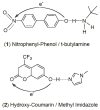
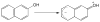
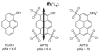

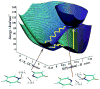
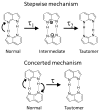

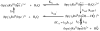
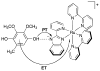
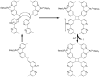
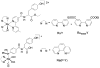


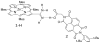
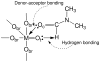
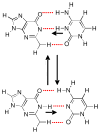
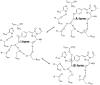

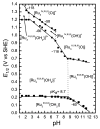
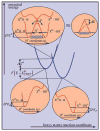
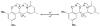
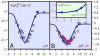
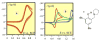
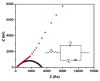
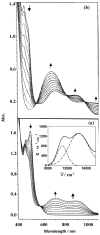
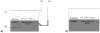
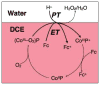

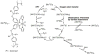
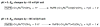
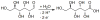
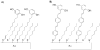

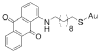
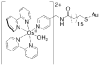
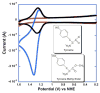

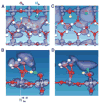
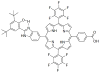

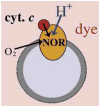

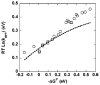

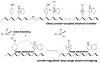
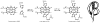
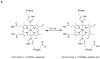


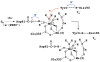

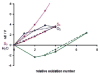




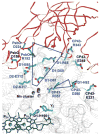

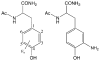
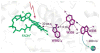
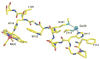
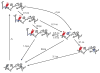

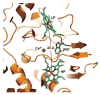
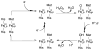
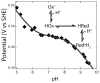

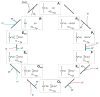
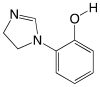
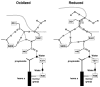


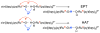
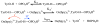




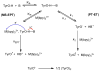




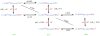
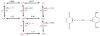
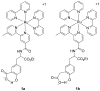
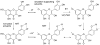
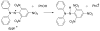
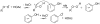
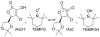
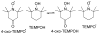
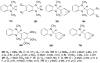

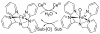
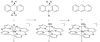
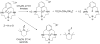
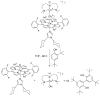
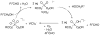

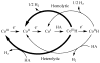
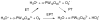
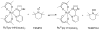


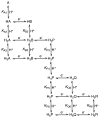
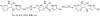
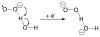

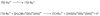
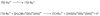
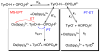
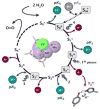
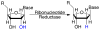
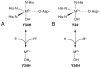
References
-
- Costentin C, Robert M, Saveant JM. Chem Rev. 2010;110:PR1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

