Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin
- PMID: 17999964
- PMCID: PMC2224738
- DOI: 10.1128/AAC.00874-07
Mefloquine-induced disruption of calcium homeostasis in mammalian cells is similar to that induced by ionomycin
Abstract
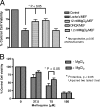
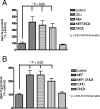
In previous studies, we have shown that mefloquine disrupts calcium homeostasis in neurons by depletion of endoplasmic reticulum (ER) stores, followed by an influx of external calcium across the plasma membrane. In this study, we explore two hypotheses concerning the mechanism(s) of action of mefloquine. First, we investigated the possibility that mefloquine activates non-N-methyl-d-aspartic acid receptors and the inositol phosphate 3 (IP3) signaling cascade leading to ER calcium release. Second, we compared the disruptive effects of mefloquine on calcium homeostasis to those of ionomycin in neuronal and nonneuronal cells. Ionomycin is known to discharge the ER calcium store (through an undefined mechanism), which induces capacitative calcium entry (CCE). In radioligand binding assays, mefloquine showed no affinity for the known binding sites of several glutamate receptor subtypes. The pattern of neuroprotection induced by a panel of glutamate receptor antagonists was dissimilar to that of mefloquine. Both mefloquine and ionomycin exhibited dose-related and qualitatively similar disruptions of calcium homeostasis in both neurons and macrophages. The influx of external calcium was blocked by the inhibitors of CCE in a dose-related fashion. Both mefloquine and ionomycin upregulated the IP3 pathway in a manner that we interpret to be secondary to CCE. Collectively, these data suggest that mefloquine does not activate glutamate receptors and that it disrupts calcium homeostasis in mammalian cells in a manner similar to that of ionomycin.
Figures

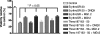

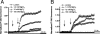

References
-
- Aarnoudse, A. L., R. H. van Schaik, J. Dieleman, M. Molokhia, M. M. van Riemsdijk, R. J. Ligthelm, D. Overbosch, I. P. van der Heiden, and B. H. Stricker. 2006. MDR1 gene polymorphisms are associated with neuropsychiatric adverse effects of mefloquine. Clin. Pharmacol. Ther. 80:367-374. - PubMed
-
- Agus, Z. S., E. Kelepouris, I. Dukes, and M. Morad. 1989. Cytosolic magnesium modulates calcium channel activity in mammalian ventricular cells. Am. J. Physiol. 256:C452-C455. - PubMed
-
- Anonymous. 1996. Guide for the care and use of laboratory animals. National Research Council, Washington, DC.
-
- Baudry, S., Y. T. Pham, B. Baune, S. Vidrequin, C. Crevoisier, F. Gimenez, and R. Farinotti. 1997. Stereoselective passage of mefloquine through the blood-brain barrier in the rat. J. Pharm. Pharmacol. 49:1086-1090. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

