Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic
- PMID: 17999970
- PMCID: PMC2224776
- DOI: 10.1128/AAC.00836-07
Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic
Abstract
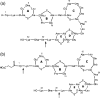
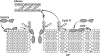
The increasing resistance of human pathogens to conventional antibiotics presents a growing threat to the chemotherapeutic management of infectious diseases. The lanthionine antibiotics, still unused as therapeutic agents, have recently attracted significant scientific interest as models for targeting and management of bacterial infections. We investigated the action of one member of this class, subtilin, which permeabilizes lipid membranes in a lipid II-dependent manner and binds bactoprenyl pyrophosphate, akin to nisin. The role the C and N termini play in target recognition was investigated in vivo and in vitro by using the natural N-terminally succinylated subtilin as well as enzymatically truncated subtilin variants. Fluorescence dequenching experiments show that subtilin induces leakage in membranes in a lipid II-dependent manner and that N-succinylated subtilin is roughly 75-fold less active. Solid-state nuclear magnetic resonance was used to show that subtilin forms complexes with membrane isoprenyl pyrophosphates. Activity assays in vivo show that the N terminus of subtilin plays a critical role in its activity. Succinylation of the N terminus resulted in a 20-fold decrease in its activity, whereas deletion of N-terminal Trp abolished activity altogether.
Figures




Similar articles
-
Lipid II-based antimicrobial activity of the lantibiotic plantaricin C.Appl Environ Microbiol. 2006 Apr;72(4):2809-14. doi: 10.1128/AEM.72.4.2809-2814.2006. Appl Environ Microbiol. 2006. PMID: 16597986 Free PMC article.
-
Interaction of type A lantibiotics with undecaprenol-bound cell envelope precursors.Microb Drug Resist. 2012 Jun;18(3):261-70. doi: 10.1089/mdr.2011.0242. Epub 2012 Mar 20. Microb Drug Resist. 2012. PMID: 22432708
-
A lesson in efficient killing from two-component lantibiotics.Mol Microbiol. 2006 Jul;61(2):271-3. doi: 10.1111/j.1365-2958.2006.05239.x. Epub 2006 Jun 12. Mol Microbiol. 2006. PMID: 16771845 Review.
-
Environmental and dynamic effects explain how nisin captures membrane-bound lipid II.Sci Rep. 2020 Jun 1;10(1):8821. doi: 10.1038/s41598-020-65522-y. Sci Rep. 2020. PMID: 32483218 Free PMC article.
-
Expanding role of lipid II as a target for lantibiotics.Future Microbiol. 2007 Oct;2(5):513-25. doi: 10.2217/17460913.2.5.513. Future Microbiol. 2007. PMID: 17927474 Review.
Cited by
-
Manipulation of charged residues within the two-peptide lantibiotic lacticin 3147.Microb Biotechnol. 2010 Mar;3(2):222-34. doi: 10.1111/j.1751-7915.2009.00145.x. Epub 2009 Aug 23. Microb Biotechnol. 2010. PMID: 21255322 Free PMC article.
-
Cell Wall-active Bacteriocins and Their Applications Beyond Antibiotic Activity.Probiotics Antimicrob Proteins. 2012 Dec;4(4):259-72. doi: 10.1007/s12602-012-9116-9. Probiotics Antimicrob Proteins. 2012. PMID: 26782186
-
Regulation of heterologous subtilin production in Bacillus subtilis W168.Microb Cell Fact. 2022 Apr 7;21(1):57. doi: 10.1186/s12934-022-01782-9. Microb Cell Fact. 2022. PMID: 35392905 Free PMC article.
-
Microbiota and gut neuropeptides: a dual action of antimicrobial activity and neuroimmune response.Psychopharmacology (Berl). 2019 May;236(5):1597-1609. doi: 10.1007/s00213-019-05224-0. Epub 2019 Apr 17. Psychopharmacology (Berl). 2019. PMID: 30997526 Free PMC article. Review.
-
An Overview of Biofilm Formation-Combating Strategies and Mechanisms of Action of Antibiofilm Agents.Life (Basel). 2022 Jul 23;12(8):1110. doi: 10.3390/life12081110. Life (Basel). 2022. PMID: 35892912 Free PMC article. Review.
References
-
- Barna, J. C. J., and D. H. Williams. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 38:339-357. - PubMed
-
- Bartlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. - PubMed
-
- Bonev, B. B., E. Breukink, E. Swiezewska, B. De Kruijff, and A. Watts. 2004. Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J. 18:1862-1869. - PubMed
-
- Bonev, B. B., W. C. Chan, B. W. Bycroft, G. C. K. Roberts, and A. Watts. 2000. Interaction of the lantibiotic nisin with mixed lipid bilayers: a P-31 and H-2 NMR study. Biochemistry 39:11425-11433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

