Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic
- PMID: 17999970
- PMCID: PMC2224776
- DOI: 10.1128/AAC.00836-07
Molecular mechanism of target recognition by subtilin, a class I lanthionine antibiotic
Abstract
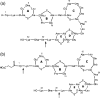
The increasing resistance of human pathogens to conventional antibiotics presents a growing threat to the chemotherapeutic management of infectious diseases. The lanthionine antibiotics, still unused as therapeutic agents, have recently attracted significant scientific interest as models for targeting and management of bacterial infections. We investigated the action of one member of this class, subtilin, which permeabilizes lipid membranes in a lipid II-dependent manner and binds bactoprenyl pyrophosphate, akin to nisin. The role the C and N termini play in target recognition was investigated in vivo and in vitro by using the natural N-terminally succinylated subtilin as well as enzymatically truncated subtilin variants. Fluorescence dequenching experiments show that subtilin induces leakage in membranes in a lipid II-dependent manner and that N-succinylated subtilin is roughly 75-fold less active. Solid-state nuclear magnetic resonance was used to show that subtilin forms complexes with membrane isoprenyl pyrophosphates. Activity assays in vivo show that the N terminus of subtilin plays a critical role in its activity. Succinylation of the N terminus resulted in a 20-fold decrease in its activity, whereas deletion of N-terminal Trp abolished activity altogether.
Figures




References
-
- Barna, J. C. J., and D. H. Williams. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 38:339-357. - PubMed
-
- Bartlett, G. R. 1959. Phosphorus assay in column chromatography. J. Biol. Chem. 234:466-468. - PubMed
-
- Bonev, B. B., E. Breukink, E. Swiezewska, B. De Kruijff, and A. Watts. 2004. Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB J. 18:1862-1869. - PubMed
-
- Bonev, B. B., W. C. Chan, B. W. Bycroft, G. C. K. Roberts, and A. Watts. 2000. Interaction of the lantibiotic nisin with mixed lipid bilayers: a P-31 and H-2 NMR study. Biochemistry 39:11425-11433. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

