Data growth and its impact on the SCOP database: new developments
- PMID: 18000004
- PMCID: PMC2238974
- DOI: 10.1093/nar/gkm993
Data growth and its impact on the SCOP database: new developments
Abstract
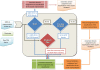
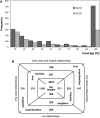
The Structural Classification of Proteins (SCOP) database is a comprehensive ordering of all proteins of known structure, according to their evolutionary and structural relationships. The SCOP hierarchy comprises the following levels: Species, Protein, Family, Superfamily, Fold and Class. While keeping the original classification scheme intact, we have changed the production of SCOP in order to cope with a rapid growth of new structural data and to facilitate the discovery of new protein relationships. We describe ongoing developments and new features implemented in SCOP. A new update protocol supports batch classification of new protein structures by their detected relationships at Family and Superfamily levels in contrast to our previous sequential handling of new structural data by release date. We introduce pre-SCOP, a preview of the SCOP developmental version that enables earlier access to the information on new relationships. We also discuss the impact of worldwide Structural Genomics initiatives, which are producing new protein structures at an increasing rate, on the rates of discovery and growth of protein families and superfamilies. SCOP can be accessed at http://scop.mrc-lmb.cam.ac.uk/scop.
Figures
References
-
- Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J. Mol. Biol. 1995;247:536–540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

