VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade
- PMID: 18000042
- PMCID: PMC2141784
- DOI: 10.1073/pnas.0708865104
VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade
Abstract
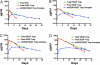
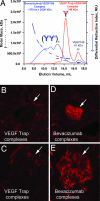
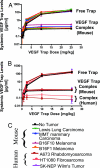
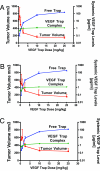
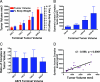
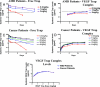
VEGF is the best characterized mediator of tumor angiogenesis. Anti-VEGF agents have recently demonstrated impressive efficacy in human cancer trials, but the optimal dosing of such agents must still be determined empirically, because biomarkers to guide dosing have yet to be established. The widely accepted (but unverified) assumption that VEGF production is quite low in normal adults led to the notion that increased systemic VEGF levels might quantitatively reflect tumor mass and angiogenic activity. We describe an approach to determine host and tumor production of VEGF, using a high-affinity and long-lived VEGF antagonist now in clinical trials, the VEGF Trap. Unlike antibody complexes that are usually rapidly cleared, the VEGF Trap forms inert complexes with tissue- and tumor-derived VEGF that remain stably in the systemic circulation, where they are readily assayable, providing unprecedented capability to accurately measure VEGF production. We report that VEGF production is surprisingly high in non-tumor-bearing rodents and humans, challenging the notion that systemic VEGF levels can serve as a sensitive surrogate for tumor load; tumor VEGF contribution becomes significant only with very large tumor loads. These findings have the important corollary that anti-VEGF therapies must be sufficiently dosed to avoid diversion by host-derived VEGF. We further show that our assay can indicate when VEGF is optimally blocked; such biomarkers to guide dosing do not exist for other anti-VEGF agents. Based on this assay, VEGF Trap doses currently being assessed in clinical trials are in the efficacious range.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

