Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group
- PMID: 18000167
- PMCID: PMC2214754
- DOI: 10.1182/blood-2007-04-084293
Outcomes in CCG-2961, a children's oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children's oncology group
Abstract
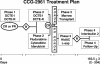
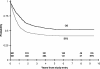
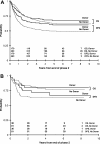
CCG-2961 incorporated 3 new agents, idarubicin, fludarabine and interleukin-2, into a phase 3 AML trial using intensive-timing remission induction/consolidation and related donor marrow transplantation or high-dose cytarabine intensification. Among 901 patients under age 21 years, 5-year survival was 52%, and event-free survival was 42%. Survival improved from 44% between 1996 and 1998 to 58% between 2000 and 2002 (P = .005), and treatment-related mortality declined from 19% to 12% (P = .025). Partial replacement of daunomycin with idarubicin in the 5-drug induction combination achieved a remission rate of 88%, similar to historical controls. Postremission survival was 56% in patients randomized to either 5-drug reinduction or fludarabine/cytarabine/idarubicin. For patients with or without a related donor, respective 5-year disease-free survival was 61% and 50% (P = .021); respective survival was 68% and 62% (P = .425). Donor availability conferred no benefit on those with inv(16) or t(8;21) cytogenetics. After cytarabine intensification, patients randomized to interleukin-2 or none experienced similar outcomes. Factors predictive of inferior survival were age more than 16 years, non-white ethnicity, absence of related donor, obesity, white blood cell count more than 100 000 x 10(9)/L, -7/7q-, -5/5q-, and/or complex karyotype. No new agent improved outcomes; experience may have contributed to better results time.
Figures

References
-
- Creutzig U, Zimmermann M, Ritter J, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. - PubMed
-
- Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. - PubMed
-
- Lie SO, Abrahamsson J, Clausen N, et al. Long-term results in children with AML: NOPHO-AML Study Group: report of three consecutive trials. Leukemia. 2005;19:2090–2100. - PubMed
-
- Entz-Werle N, Suciu S, van der Werff ten Bosch J, et al. Results of 58872 and 58921 trials in acute myeloblastic leukemia and relative value of chemotherapy vs allogeneic bone marrow transplantation in first complete remission: the EORTC Children Leukemia Group report. Leukemia. 2005;19:2072–2081. - PubMed
-
- Perel Y, Auvrignon A, Leblanc T, et al. Treatment of childhood acute myeloblastic leukemia: dose intensification improves outcome and maintenance therapy is of no benefit—multicenter studies of the French LAME (Leucemie Aigue Myeloblastique Enfant) Cooperative Group. Leukemia. 2005;19:2082–2089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

