TLR8: an innate immune receptor in brain, neurons and axons
- PMID: 18000403
- PMCID: PMC4316738
- DOI: 10.4161/cc.6.23.5018
TLR8: an innate immune receptor in brain, neurons and axons
Abstract
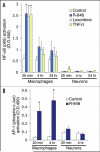
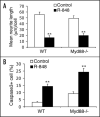
Toll-like receptors (TLRs) play essential roles in generating innate immune responses, and are evolutionarily conserved across species. In mammals, TLRs specifically recognize the conserved microbial structural motifs referred to as pathogen-associated molecular patterns (PAMPs). Ligand recognition by TLRs activates signaling cascades that culminate in proinflammatory cytokine production and eventual elimination of invading pathogens. Although TLRs in mammals are expressed predominantly in the immune system, certain TLRs with poorly characterized function are also found in neurons. We recently profiled TLR8 expression during mouse brain development and established its localization in neurons and axons. We uncovered a novel role for TLR8 as a suppressor of neurite outgrowth as well as an inducer of neuronal apoptosis, and found that TLR8 functions in neurons through an NF-kappaB-independent mechanism. These findings add a new layer of complexity for TLR signaling, and expand the realm of mammalian TLR function to the CNS beyond the originally discovered immune context. Herein, we complement our earlier report with additional data, discuss their biological and mechanistic implications in central nervous system (CNS) developmental and pathological processes, and thus further our perspective on TLR signaling and potential physiological roles in mammals.
Figures






References
-
- Lemaitre B, Nicolas E, Michaut L, Reichart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
