A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women
- PMID: 18000538
- PMCID: PMC2048661
- DOI: 10.1371/journal.pone.0001166
A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine-pyrimethamine as treatment for malaria in pregnant women
Abstract
Objective: New anti-malarial regimens are urgently needed in sub-Saharan Africa because of the increase in drug resistance. We investigated the safety and efficacy of azithromycin or artesunate combined with sulfadoxine-pyrimethamine used for treatment of malaria in pregnant women in Blantyre, Malawi.
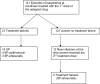
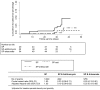
Methods/findings: This was a randomized open-label clinical trial, conducted at two rural health centers in Blantyre district, Malawi. A total of 141 pregnant women with uncomplicated Plasmodium falciparum malaria were recruited and randomly allocated to 3 treatment groups: sulfadoxine-pyrimethamine (SP; 3 tablets, 500 mg sulfadoxine and 25 mg pyrimethamine per tablet); SP plus azithromycin (1 g/dayx2 days); or SP plus artesunate (200 mg/dayx3 days). Women received two doses administered at least 4 weeks apart. Heteroduplex tracking assays were performed to distinguish recrudescence from new infections. Main outcome measures were incidence of adverse outcomes, parasite and fever clearance times and recrudescence rates. All treatment regimens were well tolerated. Two women vomited soon after ingesting azithromycin. The parasite clearance time was significantly faster in the SP-artesunate group. Recrudescent episodes of malaria were less frequent with SP-azithromycin [Hazard Ratio 0.19 (95% confidence interval 0.06 to 0.63)] and SP-artesunate [Hazard Ratio 0.25 (95% confidence interval 0.10 to 0.65)] compared with SP monotherapy. With one exception (an abortion in the SP-azithromycin group), all adverse pregnancy outcomes could be attributed to known infectious or obstetrical causes. Because of the small sample size, the effect on birth outcomes, maternal malaria or maternal anemia could not be evaluated.
Conclusions: Both SP-artesunate and SP-azithromycin appeared to be safe, well tolerated and efficacious for the treatment of malaria during pregnancy. A larger study is needed to determine their safety and efficacy in preventing poor birth outcomes.
Trial registration: ClinialTrials.gov NCT00287300.
Conflict of interest statement
Figures
References
-
- World Health Organization (WHO) 2006. World Malaria Report, 2005. Accessed at http://rbm.who.int/wmr2005.
-
- McGready R, Ashley EA, Moo E, Cho T, Barends M, et al. A randomized comparison of artesunate-atovaquone-proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. The Journal of infectious diseases. 2005;192(5):846–853. - PubMed
-
- Guyatt HL, Snow RW. The epidemiology and burden of Plasmodium falciparum-related anemia among pregnant women in sub-Saharan Africa. The American Journal of Tropical Medicine and Hygiene. 2001;64(1–2 Suppl):36–44. - PubMed
-
- Shulman CE. Malaria in pregnancy: its relevance to safe-motherhood programmes. Annals of Tropical Medicine and Parasitology. 1999;93(Suppl 1):S59–66. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

