NF-kappaB/Rel-mediated regulation of the neural fate in Drosophila
- PMID: 18000549
- PMCID: PMC2064963
- DOI: 10.1371/journal.pone.0001178
NF-kappaB/Rel-mediated regulation of the neural fate in Drosophila
Abstract
Two distinct roles are described for Dorsal, Dif and Relish, the three NF-kappaB/Rel proteins of Drosophila, in the development of the peripheral nervous system. First, these factors regulate transcription of scute during the singling out of sensory organ precursors from clusters of cells expressing the proneural genes achaete and scute. This effect is possibly mediated through binding sites for NF-kappaB/Rel proteins in a regulatory module of the scute gene required for maintenance of scute expression in precursors as well as repression in cells surrounding precursors. Second, genetic evidence suggests that the receptor Toll-8, Relish, Dif and Dorsal, and the caspase Dredd pathway are active over the entire imaginal disc epithelium, but Toll-8 expression is excluded from sensory organ precursors. Relish promotes rapid turnover of transcripts of the target genes scute and asense through an indirect, post-transcriptional mechanism. We propose that this buffering of gene expression levels serves to keep the neuro-epithelium constantly poised for neurogenesis.
Conflict of interest statement
Figures

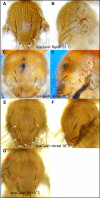
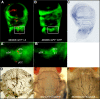
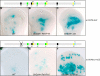

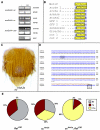

References
-
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nature Reviews Neuroscience. 2002;3:517–530. - PubMed
-
- Calleja M, Renaud O, Usui K, Pistillo D, Morata G, et al. How to pattern an epithelium: lessons from achaete-scute regulation on the notum of Drosophila. Gene. 2002;292:1–12. - PubMed
-
- Cubas P, de Celis JF, Campuzano S, Modolell J. Proneural clusters of achaete-scute expression and the generation of sensory organs in the Drosophila imaginal wing disc. GenesDev. 1991;5:996–1008. - PubMed
-
- Skeath JB, Carroll SB. Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev. 1991;5:984–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

