RNA is an integral component of chromatin that contributes to its structural organization
- PMID: 18000552
- PMCID: PMC2063516
- DOI: 10.1371/journal.pone.0001182
RNA is an integral component of chromatin that contributes to its structural organization
Abstract
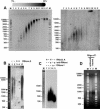
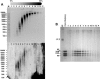
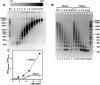
Chromatin structure is influenced by multiples factors, such as pH, temperature, nature and concentration of counterions, post-translational modifications of histones and binding of structural non-histone proteins. RNA is also known to contribute to the regulation of chromatin structure as chromatin-induced gene silencing was shown to depend on the RNAi machinery in S. pombe, plants and Drosophila. Moreover, both in Drosophila and mammals, dosage compensation requires the contribution of specific non-coding RNAs. However, whether RNA itself plays a direct structural role in chromatin is not known. Here, we report results that indicate a general structural role for RNA in eukaryotic chromatin. RNA is found associated to purified chromatin prepared from chicken liver, or cultured Drosophila S2 cells, and treatment with RNase A alters the structural properties of chromatin. Our results indicate that chromatin-associated RNAs, which account for 2%-5% of total chromatin-associated nucleic acids, are polyA(-) and show a size similar to that of the DNA contained in the corresponding chromatin fragments. Chromatin-associated RNA(s) are not likely to correspond to nascent transcripts as they are also found bound to chromatin when cells are treated with alpha-amanitin. After treatment with RNase A, chromatin fragments of molecular weight >3.000 bp of DNA showed reduced sedimentation through sucrose gradients and increased sensitivity to micrococcal nuclease digestion. This structural transition, which is observed both at euchromatic and heterochromatic regions, proceeds without loss of histone H1 or any significant change in core-histone composition and integrity.
Conflict of interest statement
Figures





References
-
- Kornberg RD. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. - PubMed
-
- McGhee JD, Felsenfeld G. Nucleosome structure. Annu Rev Biochem. 1980;49:1115–1156. - PubMed
-
- Finch T, Lutter LC, Rhodes D, Brown AS, Rushton B, et al. Structure of nucleosome core particle. Nature (London) 1977;269:29–36. - PubMed
-
- Richmond TJ, Finch JT, Rushton B, Rhodes D, Klug A. Structure of nucleosome core particle at 7Å resolution. Nature (London) 1984;311:532–537. - PubMed
-
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleoosme core particle at 2,8Å resolution. Nature. 1997;389:251–260. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

