Ligand-modified aminobisphosphonate for linking proteins to hydroxyapatite and bone surface
- PMID: 18001076
- PMCID: PMC3247143
- DOI: 10.1021/bc700196q
Ligand-modified aminobisphosphonate for linking proteins to hydroxyapatite and bone surface
Abstract
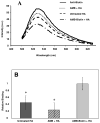
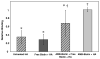
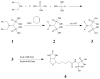
An increase in bone resorption is one of the main symptoms of osteoporosis, a disease that affects more and more individuals every day. Bisphosphonates are known to inhibit bone resorption and thus are being used as a treatment for osteoporosis. Aminobisphosphonates present a functionality that can be easily used for conjugation to other molecules, such as peptides, proteins, and ligands for protein recognition. In this study, an aminobisphosphonate conjugated with biotin was used as a model linker for protein attachment to bone. With this system, the interaction of biotinylated aminobisphosphonate with hydroxyapatite, a major mineral component of bone, was investigated. Quantification of the binding of aminobisphosphonate to hydroxyapatite was performed using a fluorescently labeled antibody for biotin. Additionally, the interaction of the biotinylated aminobisphosphonate with multiple treatments of cortical bone from the midshaft of a cow femur was studied. It was demonstrated that modified aminobisphosphonate reagents can bind hydroxyapatite and bone at high levels, while the biotin functionality is free to be recognized by the fluorescently labeled antibiotin antibody, suggesting that modified aminobisphosphonates could be used to link other peptides or proteins to the bone surface.
Figures



References
-
- Kiberstis P, Smith O, Norman C. Bone health in the balance. Science. 2000;289:1497.
-
- Ducy P, Schinke T, Karsenty G. The osteoblast: A sophisticated fibroblast under central surveillance. Science. 2000;289:1501–1504. - PubMed
-
- Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. - PubMed
-
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. - PubMed
-
- Finkelstein JS. Calcium plus vitamin D for postmenopausal women -bone appetit? N Engl J Med. 2006;354:750–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

