SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion
- PMID: 18003739
- PMCID: PMC2880884
- DOI: 10.1242/dev.010678
SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion
Abstract
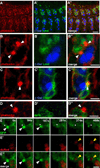
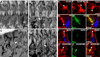
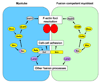
Myoblast fusion is crucial for formation and repair of skeletal muscle. Here we show that active remodeling of the actin cytoskeleton is essential for fusion in Drosophila. Using live imaging, we have identified a dynamic F-actin accumulation (actin focus) at the site of fusion. Dissolution of the actin focus directly precedes a fusion event. Whereas several known fusion components regulate these actin foci, others target additional behaviors required for fusion. Mutations in kette/Nap1, an actin polymerization regulator, lead to enlarged foci that do not dissolve, consistent with the observed block in fusion. Kette is required to positively regulate SCAR/WAVE, which in turn activates the Arp2/3 complex. Mutants in SCAR and Arp2/3 have a fusion block and foci phenotype, suggesting that Kette-SCAR-Arp2/3 participate in an actin polymerization event required for focus dissolution. Our data identify a new paradigm for understanding the mechanisms underlying fusion in myoblasts and other tissues.
Figures




References
-
- Abmayr SM, Balagopalan L, Galletta BJ, Hong SJ. Cell and molecular biology of myoblast fusion. Int Rev Cytol. 2003;225:33–89. - PubMed
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
-
- Artero RD, Castanon I, Baylies MK. The immunoglobulin-like protein Hibris functions as a dose-dependent regulator of myoblast fusion and is differentially controlled by Ras and Notch signaling. Development. 2001;128:4251–4264. - PubMed
-
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques. 2000;29:726. 728, 730, 732. - PubMed
-
- Barolo S, Castro B, Posakony JW. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques. 2004;36:436–440. 442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

