Proinflammatory effects of advanced lipoxidation end products in monocytes
- PMID: 18003754
- PMCID: PMC2695452
- DOI: 10.2337/db07-1204
Proinflammatory effects of advanced lipoxidation end products in monocytes
Abstract
Objective: The reactions of carbohydrate- or lipid-derived intermediates with proteins lead to the formation of Maillard reaction products, which subsequently leads to the formation of advanced glycation/lipoxidation end products (AGE/ALEs). Levels of AGE/ALEs are increased in diseases like diabetes. Unlike AGEs, very little is known about ALE effects in vitro. We hypothesized that ALEs can have proinflammatory effects in monocytes.
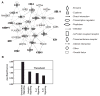
Research design and methods: In a profiling approach, conditioned media from THP-1 cells either cultured in normal glucose (5.5 mmol/l) or treated with MDA-Lys or MDA alone were hybridized to arrays containing antibodies to 120 known human cytokines/chemokines. Pathway analyses with bioinformatics software were used to identify signalling networks.
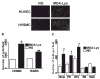
Results: Synthetic ALE (malondialdehyde-lysine [MDA-Lys]) (50 micromol/l) could induce oxidant stress and also activate the transcriptional factor nuclear factor-kappaB (NF-kappaB) in THP-1 monocytes. MDA-Lys also significantly increased the expression of key candidate proinflammatory genes, interferon-gamma-inducible protein-10, beta1- and beta2-integrins, cyclooxygenase-2 (COX-2), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 and -8, and inducible nitric-oxide synthase, which are also associated with monocyte dysfunction. Several key target proinflammatory proteins were significantly induced by MDA-Lys relative to normal glucose or MDA alone, including MCP-1; tumor necrosis factor ligand superfamily member-14; chemokine CC motif ligand-11 (CCL11); growth-related oncogene-alpha, -beta, and -gamma; and chemokine CXC motif ligand-13. Bioinformatics analyses identified a network of chemokine signaling among MDA-Lys-regulated genes. MDA-Lys also increased monocyte binding to vascular smooth muscle and endothelial cells. Furthermore, plasma from diabetic rats showed significantly higher levels of MDA-Lys and CCL11.
Conclusions: These new results suggest that ALEs can promote monocyte activation and vascular complications via induction of inflammatory pathways and networks.
Figures


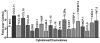


References
-
- Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25:275–281. - PubMed
-
- Portero-Otin M, Bellmunt MJ, Requena JR, et al. Protein modification by advanced Maillard adducts can be modulated by dietary polyunsaturated fatty acids. Biochem Soc Trans. 2003;31:1403–1405. - PubMed
-
- Huang GS, Wang ZP, Wang SC, et al. Intracellular generation of MDA-LYS epitope in foam cells. Life Sci. 1999;65:285–296. - PubMed
-
- Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

