The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation
- PMID: 18003853
- PMCID: PMC5724774
- DOI: 10.1523/JNEUROSCI.2522-07.2007
The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation
Abstract
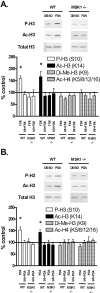
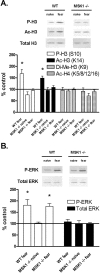
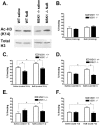
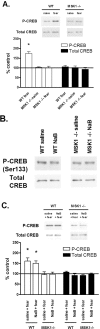
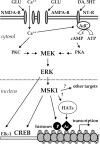
The extracellular signal-regulated kinase (ERK)/MAPK (mitogen-activated protein kinase) cascade has been established as a potent regulator of gene transcription in long-term memory formation, but the precise mechanisms of this regulation are poorly understood. ERK does not directly affect many of its nuclear targets, but rather must act through intermediary kinases. In this study, we investigated the role of mitogen- and stress-activated protein kinase 1 (MSK1), a nuclear kinase downstream of ERK, in chromatin remodeling during hippocampus-dependent memory formation. Mice lacking MSK1 show impaired Pavlovian fear conditioning and spatial learning, as well as a deficiency in histone phosphorylation and acetylation in the hippocampus after fear training. In addition, hippocampal slices from MSK1 knock-out mice exhibit a deficiency in both histone phosphorylation and acetylation after activation of the ERK pathway in vitro. In vivo injections of a histone deacetylase inhibitor, sodium butyrate, fail to alleviate the fear conditioning deficit in MSK1 knock-out mice. Finally, MSK1 knock-out mice demonstrate a deficiency in cAMP response element-binding protein (CREB) phosphorylation after fear training, which persists after sodium butyrate injection. This suggests that CREB phosphorylation and histone acetylation represent parallel targets of MSK1 function. Our study identifies MSK1 as an important regulator of chromatin remodeling in long-term memory.
Figures
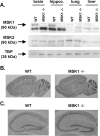
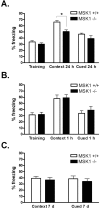
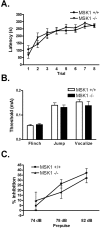
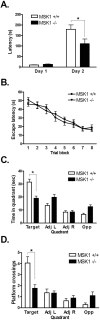
References
-
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
-
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, Kandel ER, Barco A. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. - PubMed
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
-
- Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev. 2002;12:142–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
