The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage
- PMID: 18003894
- PMCID: PMC2141848
- DOI: 10.1073/pnas.0709379104
The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage
Abstract
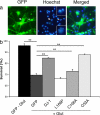
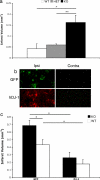
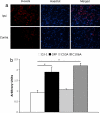
Recent evidence has indicated that common mechanisms play roles among multiple neurological diseases. However, the specifics of these pathways are not completely understood. Stroke is caused by the interruption of blood flow to the brain, and cumulative evidence supports the critical role of oxidative stress in the ensuing neuronal death process. DJ-1 (PARK7) has been identified as the gene linked to early-onset familial Parkinson's disease. Currently, our work also shows that DJ-1 is central to death in both in Vitro and in Vivo models of stroke. Loss of DJ-1 increases the sensitivity to excitotoxicity and ischemia, whereas expression of DJ-1 can reverse this sensitivity and indeed provide further protection. Importantly, DJ-1 expression decreases markers of oxidative stress after stroke insult in Vivo, suggesting that DJ-1 protects through alleviation of oxidative stress. Consistent with this finding, we demonstrate the essential role of the oxidation-sensitive cysteine-106 residue in the neuroprotective activity of DJ-1 after stroke. Our work provides an important example of how a gene seemingly specific for one disease, in this case Parkinson's disease, also appears to be central in other neuropathological conditions such as stroke. It also highlights the important commonalities among differing neuropathologies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Centers for Disease Control Prevention (CDC) MMWR Morb Mortal Wkly Rep. 2007;56:469–474. - PubMed
-
- Hunter DJW, Grant HJ, Purdue MPH, Spasoff RA, Dorland JL, Bains N. Chronic Dis Can. 2004;25:138–146. - PubMed
-
- Alexandrova M, Bochev P, Markova V, Bechev B, Popova M, Danovska M, Simeonova V. J Clin Neurosci. 2004;11:501–506. - PubMed
-
- Schaller B, Graf R. J Cereb Blood Flow Metab. 2004;24:351–371. - PubMed
-
- Adam-Vizi V. Antioxid Redox Signal. 2005;7:1140–1149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

