Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion
- PMID: 18003913
- PMCID: PMC2141843
- DOI: 10.1073/pnas.0707452104
Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion
Abstract
Herpes simplex virus entry into cells requires four glycoproteins, gB, gD, gH, and gL. Binding of gD to one of its receptors triggers steps requiring the core fusion proteins, gB and the gH/gL heterodimer. There is evidence that gH/gL initiates hemifusion of cells, but whether this complex interacts physically with gB to cause complete fusion is unknown. We used bimolecular complementation (BiMC) of enhanced yellow fluorescent protein (EYFP) to detect glycoprotein interactions during cell-cell fusion. The N- or C-terminal half of EYFP was fused to the C terminus of gD, gB, and gH to form six chimeric proteins (Dn, Dc, Bn, Bc, Hn, and Hc). BiMC was detected by confocal microscopy. Receptor-bearing (C10) cells cotransfected with Dn and Bc or Dn, Hc, and untagged gL exhibited EYFP fluorescence, indicative of interactions between gD and gB and between gD and gH/gL. EYFP complementation did not occur in cells transfected with gL, Bc, and Hn. However, when gD was coexpressed with these other three proteins, cell-cell fusion occurred and the syncytia exhibited bright EYFP fluorescence. To separate glycoprotein expression from fusion, we transfected C10 cells with gL, Bc, and Hn for 20 h and then added soluble gD to trigger fusion. We detected fluorescent syncytia within 10 min, and both their number and size increased with exposure time to gD. Thus, when gD binds its receptor, the core fusion machinery is triggered to form a multiprotein complex as a step in fusion and possibly virus entry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



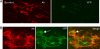
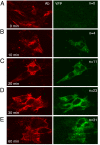
References
-
- Wu SR, Haag L, Hammar L, Wu B, Garoff H, Xing L, Murata K, Cheng RH. J Biol Chem. 2007;282:6752–6762. - PubMed
-
- Spear PG, Eisenberg RJ, Cohen GH. Virology. 2000;275:1–8. - PubMed
-
- Krummenacher C, Carfi A, Eisenberg RJ, Cohen GH. In: Viral Entry into Host Cells. Pohlmann S, Simmons G, editors. Austin, TX: Landes Bioscience; 2007. in press.
-
- Spear PG, Manoj S, Yoon M, Jogger CR, Zago A, Myscofski D. Virology. 2006;344:17–24. - PubMed
-
- Roche S, Bressanelli S, Rey FA, Gaudin Y. Science. 2006;313:187–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

