Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings
- PMID: 18003924
- PMCID: PMC2141859
- DOI: 10.1073/pnas.0705082104
Separate functions for nuclear and cytoplasmic cryptochrome 1 during photomorphogenesis of Arabidopsis seedlings
Abstract
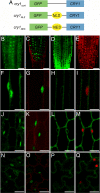
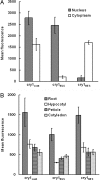
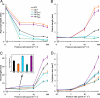
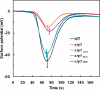
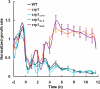
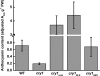
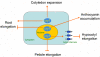
Cryptochrome blue-light receptors mediate many aspects of plant photomorphogenesis, such as suppression of hypocotyl elongation and promotion of cotyledon expansion and root growth. The cryptochrome 1 (cry1) protein of Arabidopsis is present in the nucleus and cytoplasm of cells, but how the functions of one pool differ from the other is not known. Nuclear localization and nuclear export signals were genetically engineered into GFP-tagged cry1 molecules to manipulate cry1 subcellular localization in a cry1-null mutant background. The effectiveness of the engineering was confirmed by confocal microscopy. The ability of nuclear or cytoplasmic cry1 to rescue a variety of cry1 phenotypes was determined. Hypocotyl growth suppression by blue light was assessed by standard end-point analyses and over time with high resolution by a custom computer-vision technique. Both assays indicated that nuclear, rather than cytoplasmic, cry1 was the effective molecule in these growth inhibitions, as was the case for the mechanistically linked membrane depolarization, which occurs within several seconds of cry1 activation. Petiole elongation also was inhibited by nuclear, but not cytoplasmic, cry1. Conversely, primary root growth and cotyledon expansion in blue light were promoted by cytoplasmic cry1 and inhibited by nuclear cry1. Anthocyanin production in response to blue light was strongly stimulated by nuclear cry1 and, to a lesser extent, by cytoplasmic cry1. An important step toward elucidation of cry1 signaling pathways is the recognition that different subcellular pools of the photoreceptor have different functions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

