Adducin promotes micrometer-scale organization of beta2-spectrin in lateral membranes of bronchial epithelial cells
- PMID: 18003973
- PMCID: PMC2230604
- DOI: 10.1091/mbc.e07-08-0818
Adducin promotes micrometer-scale organization of beta2-spectrin in lateral membranes of bronchial epithelial cells
Abstract
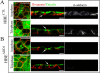
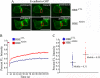
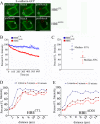
Adducin promotes assembly of spectrin-actin complexes, and is a target for regulation by calmodulin, protein kinase C, and rho kinase. We demonstrate here that adducin is required to stabilize preformed lateral membranes of human bronchial epithelial (HBE) cells through interaction with beta2-spectrin. We use a Tet-on regulated inducible small interfering RNA (siRNA) system to deplete alpha-adducin from confluent HBE cells. Depletion of alpha-adducin resulted in increased detergent solubility of spectrin after normal membrane biogenesis during mitosis. Conversely, depletion of beta2-spectrin resulted in loss of adducin from the lateral membrane. siRNA-resistant alpha-adducin prevented loss of lateral membrane, but only if alpha-adducin retained the MARCKS domain that mediates spectrin-actin interactions. Phospho-mimetic versions of adducin with S/D substitutions at protein kinase C phosphorylation sites in the MARCKS domain were not active in rescue. We find that adducin modulates long-range organization of the lateral membrane based on several criteria. First, the lateral membrane of adducin-depleted cells exhibited reduced height, increased curvature, and expansion into the basal surface. Moreover, E-cadherin-GFP, which normally is restricted in lateral mobility, rapidly diffuses over distances up to 10 microm. We conclude that adducin acting through spectrin provides a novel mechanism to regulate global properties of the lateral membrane of bronchial epithelial cells.
Figures






References
-
- Bennett V., Gardner K., Steiner J. P. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J. Biol. Chem. 1988;263(12):5860–5869. - PubMed
-
- Bennett V., Baines A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81(3):1353–1392. - PubMed
-
- Chen H., et al. Combined deletion of mouse dematin-headpiece and beta-adducin exerts a novel effect on the spectrin-actin junctions leading to erythrocyte fragility and hemolytic anemia. J. Biol. Chem. 2007;282(6):4124–4135. - PubMed
-
- Davis J. Q., Bennett V. The anion exchanger and Na+K(+)-ATPase interact with distinct sites on ankyrin in in-vitro assays. J. Biol. Chem. 1990;265(28):17252–17256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

