Intravital insights in skin wound healing using the mouse dorsal skin fold chamber
- PMID: 18005122
- PMCID: PMC2375850
- DOI: 10.1111/j.1469-7580.2007.00822.x
Intravital insights in skin wound healing using the mouse dorsal skin fold chamber
Abstract
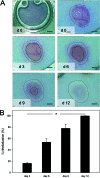
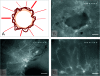
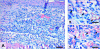
The skin fold chamber is one of the most accepted animal models for studying the microcirculation both in health and disease. Here we describe for the first time the alternative use of the skin fold chamber in mice for intravital microscopic investigation of skin regeneration after creating a full dermal thickness wound. The dorsal skin fold chamber was implanted in hairless SKH1-hr mice and a full dermal thickness wound (area approximately 4 mm2) was created. By means of intravital fluorescence microscopy, the kinetics of wound healing were analyzed for 12 days post wounding with assessment of epithelialization and nutritive perfusion. The morphology of the regenerating skin was characterized by hematoxylin-eosin histology and immunohistochemistry for proliferation and microvessel density. The model allows the continuous visualization of wound closure with complete epithelialization at day 12. Furthermore, a sola cutis se reficientis could be described by an inner circular ring of vessels at the wound margin surrounded by outer radial passing vessels. Inner circular vessels presented initially with large diameters and matured towards diameters of less than 15 microm for conversion into radial spreading outer vessels. Furthermore, wound healing showed all diverse core issues of skin repair. In summary, we were able to establish a model for the analysis of microcirculation in the healing skin of the mouse. This versatile model allows distinct analysis of new vessel formation and maturation in regenerating skin as well as evaluation of skin healing under different pathologic conditions.
Figures




References
-
- Algire GH. An adaptation of the transparent-chamber technique to the mouse. J Natl Cancer Inst. 1943;4:1–11.
-
- Allgower M, Schoenenberger GA, Sparkes BG. Burning the largest immune organ. Burns. 1995;21(Suppl. 1):7–47. - PubMed
-
- Amon M, Menger MD, Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-alpha-induced microcirculatory dysfunction and apoptotic cell death. FASEB J. 2003;17:175–185. - PubMed
-
- Bondar I, Uhl E, Barker JH, Galla TJ, Hammersen F, Messmer K. A new model for studying microcirculatory changes during dermal wound healing. Res Exp Med. 1991;191:379–388. - PubMed
-
- Bordel R, Laschke MW, Menger MD, Vollmar B. Inhibition of p53 during physiological angiogenesis in the hamster ovary does not affect extent of new vessel formation but delays vessel maturation. Cell Tissue Res. 2005;320:427–435. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

