The PC12 cell as model for neurosecretion
- PMID: 18005394
- PMCID: PMC2663028
- DOI: 10.1111/j.1748-1716.2007.01805.x
The PC12 cell as model for neurosecretion
Abstract
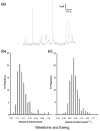
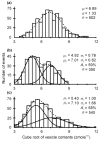
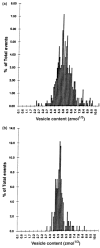
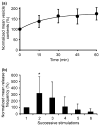
This review attempts to touch on the history and application of amperometry at PC12 cells for fundamental investigation into the exocytosis process. PC12 cells have been widely used as a model for neural differentiation and as such they have been used to examine the effects of differentiation on exocytotic release and specifically release at varicosities. In addition, dexamethasone-differentiated cells have been shown to have an increased number of releasable vesicles with increased quantal size, thereby allowing for an even broader range of applications including neuropharmacological and neurotoxicological studies. PC12 cells exhibiting large numbers of events have two distinct pools of vesicles, one about twice the quantal size of the other and each about half the total releasable vesicles. As will be outlined in this review, these cells have served as an extremely useful model of exocytosis in the study of the latency of stimulation-release coupling, the role of exocytotic proteins in regulation of release, effect of drugs on quantal size, autoreceptors, fusion pore biophysics, environmental factors, health and disease. As PC12 cells have some advantages over other models for neurosecretion, including chromaffin cells, it is more than likely that in the following decade PC12 cells will continue to serve as a model to study exocytosis.
Conflict of interest statement
Conflict of interest
There is no conflict of interest.
Figures

References
-
- Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. The exocytotic event in chromaffin cells revealed by patch amperometry. Nature. 1997;389:509–512. - PubMed
-
- Alvarez de Toledo G, Fernandez-Chacon R, Fernandez JM. Release of secretory products during transient vesicle fusion. Nature. 1993;363:554–558. - PubMed
-
- Barclay JW, Morgan A, Burgoyne RD. Calcium-dependent regulation of exocytosis. Cell Calcium. 2005;38:343–353. - PubMed
-
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

