Endometrial regenerative cells: a novel stem cell population
- PMID: 18005405
- PMCID: PMC2212625
- DOI: 10.1186/1479-5876-5-57
Endometrial regenerative cells: a novel stem cell population
Abstract
Angiogenesis is a critical component of the proliferative endometrial phase of the menstrual cycle. Thus, we hypothesized that a stem cell-like population exist and can be isolated from menstrual blood. Mononuclear cells collected from the menstrual blood contained a subpopulation of adherent cells which could be maintained in tissue culture for >68 doublings and retained expression of the markers CD9, CD29, CD41a, CD44, CD59, CD73, CD90 and CD105, without karyotypic abnormalities. Proliferative rate of the cells was significantly higher than control umbilical cord derived mesenchymal stem cells, with doubling occurring every 19.4 hours. These cells, which we termed "Endometrial Regenerative Cells" (ERC) were capable of differentiating into 9 lineages: cardiomyocytic, respiratory epithelial, neurocytic, myocytic, endothelial, pancreatic, hepatic, adipocytic, and osteogenic. Additionally, ERC produced MMP3, MMP10, GM-CSF, angiopoietin-2 and PDGF-BB at 10-100,000 fold higher levels than two control cord blood derived mesenchymal stem cell lines. Given the ease of extraction and pluripotency of this cell population, we propose ERC as a novel alternative to current stem cells sources.
Figures

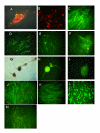

References
-
- Matikainen T, Laine J. Placenta-an alternative source of stem cells. Toxicol Appl Pharmacol. 207:544–9. 2005 Sep 1. - PubMed
-
- Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 112:I166–72. 2005 Aug 30. - PubMed
-
- Lees JG, Lim SA, Croll T, Williams G, Lui S, Cooper-White J, McQuade LR, Mathiyalagan B, Tuch BE. Transplantation of 3D scaffolds seeded with human embryonic stem cells: biological features of surrogate tissue and teratoma-forming potential. Regen Med. 2007;2:289–300. doi: 10.2217/17460751.2.3.289. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

