The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies
- PMID: 18005681
- PMCID: PMC2212603
- DOI: 10.1016/j.chom.2007.01.002
The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies
Abstract
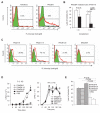
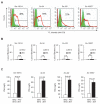
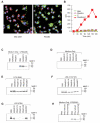
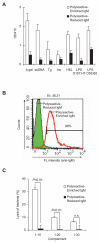
Polyreactive antibodies bind to a variety of structurally unrelated antigens. The function of these antibodies, however, has remained an enigma, and because of their low binding affinity their biological relevance has been questioned. Using a panel of monoclonal polyreactive antibodies, we showed that these antibodies can bind to both Gram-negative and Gram-positive bacteria and acting through the classical complement pathway can inhibit bacterial growth by lysis, generate anaphylatoxin C5a, enhance phagocytosis, and neutralize the functional activity of endotoxin. Polyreactive antibody-enriched, but not polyreactive antibody-reduced, IgM prepared from normal human serum displays antibacterial activity similar to that of monoclonal polyreactive IgM. We conclude that polyreactive antibodies are a major contributor to the broad antibacterial activity of the natural antibody repertoire.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

