Temporal neurotransmitter conditioning restores the functional activity of adult spinal cord neurons in long-term culture
- PMID: 18005959
- PMCID: PMC2249621
- DOI: 10.1016/j.expneurol.2007.09.019
Temporal neurotransmitter conditioning restores the functional activity of adult spinal cord neurons in long-term culture
Abstract
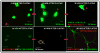
The ability to culture functional adult mammalian spinal cord neurons represents an important step in the understanding and treatment of a spectrum of neurological disorders including spinal cord injury. Previously, the limited functional recovery of these cells, as characterized by a diminished ability to initiate action potentials and to exhibit repetitive firing patterns, has arisen as a major impediment to their physiological relevance. In this report, we demonstrate that single temporal doses of the neurotransmitters serotonin, glutamate (N-acetyl-DL-glutamic acid) and acetylcholine-chloride lead to the full electrophysiological functional recovery of adult mammalian spinal cord neurons, when they are cultured under defined serum-free conditions. Approximately 60% of the neurons treated regained their electrophysiological signature, often firing single, double and, most importantly, multiple action potentials.
Figures








Similar articles
-
Changes in electrophysiological properties of lamprey spinal motoneurons during fictive swimming.J Neurophysiol. 2002 Nov;88(5):2463-76. doi: 10.1152/jn.00725.2001. J Neurophysiol. 2002. PMID: 12424286
-
Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival.Exp Neurol. 2002 Jan;173(1):46-62. doi: 10.1006/exnr.2001.7834. Exp Neurol. 2002. PMID: 11771938
-
Ethanol alters synaptic activity in cultured spinal cord neurons.Brain Res. 1982 Jul 8;243(1):25-33. doi: 10.1016/0006-8993(82)91117-9. Brain Res. 1982. PMID: 6126256
-
Muscle-conditioned media and cAMP promote survival and neurite outgrowth of adult spinal cord motor neurons.Exp Neurol. 2009 Dec;220(2):303-15. doi: 10.1016/j.expneurol.2009.09.003. Epub 2009 Sep 9. Exp Neurol. 2009. PMID: 19747480
-
Dedifferentiation of intrinsic response properties of motoneurons in organotypic cultures of the spinal cord of the adult turtle.Eur J Neurosci. 2000 Jul;12(7):2397-404. doi: 10.1046/j.1460-9568.2000.00134.x. Eur J Neurosci. 2000. PMID: 10947818
Cited by
-
Skeletal muscle tissue engineering: a maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression.Biomaterials. 2009 Oct;30(29):5392-402. doi: 10.1016/j.biomaterials.2009.05.081. Epub 2009 Jul 22. Biomaterials. 2009. PMID: 19625080 Free PMC article.
-
Microphysiological systems and low-cost microfluidic platform with analytics.Stem Cell Res Ther. 2013;4 Suppl 1(Suppl 1):S9. doi: 10.1186/scrt370. Epub 2013 Dec 20. Stem Cell Res Ther. 2013. PMID: 24565109 Free PMC article. Review.
-
Neurotropic activity and safety of methylene-cycloalkylacetate (MCA) derivative 3-(3-allyl-2-methylenecyclohexyl) propanoic acid.ACS Chem Neurosci. 2020 Sep 2;11(17):2577-2589. doi: 10.1021/acschemneuro.0c00255. Epub 2020 Aug 3. ACS Chem Neurosci. 2020. PMID: 32667774 Free PMC article.
-
Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform.Biomaterials. 2011 Jun;32(18):4267-74. doi: 10.1016/j.biomaterials.2010.12.022. Epub 2011 Mar 31. Biomaterials. 2011. PMID: 21453966 Free PMC article.
-
Electrophysiological and immunocytochemical characterization of DRG neurons on an organosilane surface in serum-free medium.In Vitro Cell Dev Biol Anim. 2008 May-Jun;44(5-6):162-8. doi: 10.1007/s11626-008-9097-x. Epub 2008 May 14. In Vitro Cell Dev Biol Anim. 2008. PMID: 18478304
References
-
- Dumont RJ, Okonkwo DO, Verma RS, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clinical Neuropharmacology. 2001;24:254–264. - PubMed
-
- Schwab ME. Repairing the injured spinal cord. Science. 2002;295:10291031. - PubMed
-
- Das M, Bhargava N, Gregory C, Riedel L, Molnar P, Hickman JJ. Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell Dev Biol Anim. 2005;41:343–348. - PubMed
-
- Brewer GJ. Regeneration and proliferation of embryonic and adult rat hippocampal neurons in culture. Exp Neurol. 1999;159:237–247. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

