Synthesis and structural characterization of 1- and 2-substituted indazoles: ester and carboxylic acid derivatives
- PMID: 18007393
- PMCID: PMC6148532
- DOI: 10.3390/11110867
Synthesis and structural characterization of 1- and 2-substituted indazoles: ester and carboxylic acid derivatives
Abstract
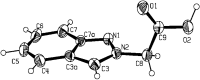
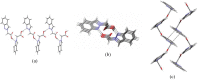
A series of indazoles substituted at the N-1 and N-2 positions with ester-containing side chains -(CH2)(n)CO2R of different lengths (n = 0-6, 9, 10) are described. Nucleophilic substitution reactions on halo esters (X(CH2)(n)CO2R) by 1H-indazole inalkaline solution lead to mixtures of N-1 and N-2 isomers, in which the N-1 isomer predominates. Basic hydrolysis of the ester derivatives allowed the synthesis of the corresponding indazole carboxylic acids. All compounds were fully characterised by multinuclear NMR and IR spectroscopies, MS spectrometry and elemental analysis; the NMR spectroscopic data were used for structural assignment of the N-1 and N-2 isomers. The molecular structure of indazol-2-yl-acetic acid (5b) was determined by X-ray diffraction, which shows a supramolecular architecture involving O2-H...N1 intermolecular hydrogen bonds.
Figures

Similar articles
-
Efficient MW-Assisted Synthesis, Spectroscopic Characterization, X-ray and Antioxidant Properties of Indazole Derivatives.Molecules. 2016 Jul 9;21(7):903. doi: 10.3390/molecules21070903. Molecules. 2016. PMID: 27409599 Free PMC article.
-
A one-step synthesis of 2,4-unsubstituted quinoline-3-carboxylic acid esters from o-nitrobenzaldehydes.J Org Chem. 2010 May 21;75(10):3488-91. doi: 10.1021/jo100392x. J Org Chem. 2010. PMID: 20420395
-
1H-indazole and 2H-indazole derivatives of androsta-5,16-dien-3beta-ol.Acta Crystallogr C. 2008 Apr;64(Pt 4):o217-9. doi: 10.1107/S0108270108005842. Epub 2008 Mar 15. Acta Crystallogr C. 2008. PMID: 18391393
-
Synthesis of Carboxylic Acids and Esters from CO2.Top Curr Chem (Cham). 2017 Feb;375(1):4. doi: 10.1007/s41061-016-0091-6. Epub 2016 Dec 12. Top Curr Chem (Cham). 2017. PMID: 27957706 Review.
-
Stereocontrolled alkylation of unsaturated compounds with alkoxytitanacyclopropane reagents.Chem Rec. 2008;8(5):269-78. doi: 10.1002/tcr.20155. Chem Rec. 2008. PMID: 18956460 Review.
Cited by
-
3-Alkoxy-1-Benzyl-5-Nitroindazole Derivatives Are Potent Antileishmanial Compounds.Int J Mol Sci. 2024 Oct 1;25(19):10582. doi: 10.3390/ijms251910582. Int J Mol Sci. 2024. PMID: 39408911 Free PMC article.
-
A General N-alkylation Platform via Copper Metallaphotoredox and Silyl Radical Activation of Alkyl Halides.Chem. 2021 Jul 8;7(7):1827-1842. doi: 10.1016/j.chempr.2021.05.005. Epub 2021 Jun 16. Chem. 2021. PMID: 34423174 Free PMC article.
-
Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives.Molecules. 2018 Oct 26;23(11):2783. doi: 10.3390/molecules23112783. Molecules. 2018. PMID: 30373212 Free PMC article. Review.
-
The Suzuki reaction applied to the synthesis of novel pyrrolyl and thiophenyl indazoles.Molecules. 2012 Apr 16;17(4):4508-21. doi: 10.3390/molecules17044508. Molecules. 2012. PMID: 22508331 Free PMC article.
-
Regioselective N-alkylation of the 1H-indazole scaffold; ring substituent and N-alkylating reagent effects on regioisomeric distribution.Beilstein J Org Chem. 2021 Aug 2;17:1939-1951. doi: 10.3762/bjoc.17.127. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34386104 Free PMC article.
References
-
-
Elguero J. In: Comprehensive Heterocyclic Chemistry: Pyrazoles and their benzo derivatives. Katritzky A. R., Rees C. W., editors. Vol. 5. Pergamon Press; Oxford: 1984. pp. 167–303. Elguero J. In: Comprehensive Heterocyclic Chemistry II: Pyrazoles. Katritzky A. R., Rees C. W., Scriven E. F. V., editors. Vol. 3. Pergamon Press; Oxford: 1996. pp. 1–75.
-
-
-
Stadlbauer W. In: Houben-Weyl, Methoden der Organischen Chemie: Indazole (Benzopyrazole) Schaumann E., editor. Vol. E8b. Georg-Thieme-Verlag Stuttgart; New York: 1994. pp. 764–864. Hetarenes III/2. Stadlbauer W. In: Science of Synthesis: Indazoles. Neier R., editor. Vol. 2.12.4. Georg-Thieme-Verlag Stuttgart; New York: 2002. pp. 227–324. Hetarenes.
-
-
- Ikeda Y., Takano N., Matsushita H., Shiraki Y., Koide T., Nagashima R., Fujimura Y., Shindo M., Suzuki S., Iwasaki T. Arzneim.-Forsch. 1979;29:511–520. - PubMed
-
- Picciola G., Ravenna F., Carenini G., Gentili P., Riva M. Farmaco Ed. Sci. 1981;36:1037–1056. - PubMed
-
- Budavari S., editor. The Merck Index. 12th ed. Merck & Co.; Rahway, New Jersey: 1996.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

