Protective immune mechanisms in helminth infection
- PMID: 18007680
- PMCID: PMC2258092
- DOI: 10.1038/nri2199
Protective immune mechanisms in helminth infection
Abstract
Important insights have recently been gained in our understanding of how host immune responses mediate resistance to parasitic helminths and control associated pathological responses. Although similar cells and cytokines are evoked in response to infection by helminths as diverse as nematodes and schistosomes, the components of the response that mediate protection are dependent on the particular parasite. In this Review, we examine recent findings regarding the mechanisms of protection in helminth infections that have been elucidated in murine models and discuss the implications of these findings in terms of future therapies.
Figures
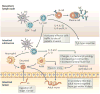

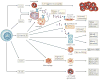
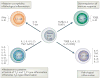
References
-
- WHO. World Health Report. World Health Organization; Geneva, Switzerland: 1999.
-
- Capron A, Riveau G, Capron M, Trottein F. Schistosomes: the road from host-parasite interactions to vaccines in clinical trials. Trends Parasitol. 2005;21:143–149. - PubMed
-
- Goud GN, et al. Expression of the Necator americanus hookworm larval antigen Na-ASP-2 in Pichia pastoris and purification of the recombinant protein for use in human clinical trials. Vaccine. 2005;23:4754–4764. - PubMed
-
- Fallon PG, Mangan NE. Suppression of TH2-type allergic reactions by helminth infection. Nature Rev Immunol. 2007;7:220–230. - PubMed
-
- Wilson MS, Maizels RM. Regulation of allergy and autoimmunity in helminth infection. Clin Rev Allergy Immunol. 2004;26:35–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

