Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells
- PMID: 18007995
- PMCID: PMC2072832
- DOI: 10.1289/ehp.10328
Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells
Abstract
Background: Polychlorinated biphenyls (PCBs) may interfere with thyroid hormone (TH) signaling by reducing TH levels in blood, by exerting direct effects on TH receptors (TRs), or both.
Objective: Our objective was to identify individual PCBs that directly affect TH signaling by acting on the TR.
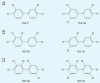
Methods: We administered a mixture of six PCB congeners based on their ortho substitution pattern, including PCBs 77 and 126 (non-ortho), PCBs 105 and 118 (mono-ortho), and PCBs 138 and 153 (di-ortho), to pregnant Sprague-Dawley rats from gestational days (G) 6 to 16. This mixture, or various combinations of the components, was also evaluated in a transient transfection system using GH3 cells.
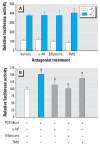
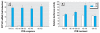
Results: The mixture reduced serum TH levels in pregnant rats on G16 but simultaneously up-regulated the expression of malic enzyme in liver. It also functioned as a TR agonist in vitro; however, none of the individual PCB congeners comprising this mixture were active in this system. Using the aryl hydrocarbon receptor (AhR) antagonist alpha-naphthoflavone, and the cytochrome P450 (CYP)1A1 antagonist ellipticine, we show that the effect of the mixture on the thyroid hormone response element required AhR and CYP1A1.
Conclusions: We propose that PCB 126 induces CYP1A1 through the AhR in GH3 cells, and that CYP1A1 activates PCB 105 and/or 118 to a form a compound that acts as a TR agonist. These data suggest that some tissues may be especially vulnerable to PCBs interfering directly with TH signaling due to their capacity to express CYP1A1 in response to coplanar PCBs (or other dioxin-like molecules) if sufficient mono-ortho PCBs are present.
Keywords: AhR; CYP1A1; PCB metabolism; endocrine disruption; thyroid hormone.
Figures
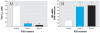
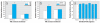
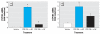
Similar articles
-
Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development.Endocrinology. 2011 Jul;152(7):2909-19. doi: 10.1210/en.2010-1490. Epub 2011 May 3. Endocrinology. 2011. PMID: 21540284 Free PMC article.
-
Antagonism of aryl hydrocarbon receptor-dependent induction of CYP1A1 and inhibition of IgM expression by di-ortho-substituted polychlorinated biphenyls.Toxicol Appl Pharmacol. 2003 Feb 15;187(1):11-21. doi: 10.1016/s0041-008x(02)00040-6. Toxicol Appl Pharmacol. 2003. PMID: 12628580
-
Divergent Effects of Dioxin- or Non-Dioxin-Like Polychlorinated Biphenyls on the Apoptosis of Primary Cell Culture from the Mouse Pituitary Gland.PLoS One. 2016 Jan 11;11(1):e0146729. doi: 10.1371/journal.pone.0146729. eCollection 2016. PLoS One. 2016. PMID: 26752525 Free PMC article.
-
PCBs: structure-function relationships and mechanism of action.Environ Health Perspect. 1985 May;60:47-56. doi: 10.1289/ehp.856047. Environ Health Perspect. 1985. PMID: 2992927 Free PMC article. Review.
-
Structure-activity relationships of potentially neurotoxic PCB congeners in the rat.Neurotoxicology. 1997;18(2):425-41. Neurotoxicology. 1997. PMID: 9291492 Review.
Cited by
-
Developmental triclosan exposure decreases maternal, fetal, and early neonatal thyroxine: a dynamic and kinetic evaluation of a putative mode-of-action.Toxicology. 2012 Oct 9;300(1-2):31-45. doi: 10.1016/j.tox.2012.05.023. Epub 2012 Jun 1. Toxicology. 2012. PMID: 22659317 Free PMC article.
-
Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro.Environ Health Perspect. 2008 Sep;116(9):1231-6. doi: 10.1289/ehp.11176. Environ Health Perspect. 2008. PMID: 18795168 Free PMC article.
-
TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis.J Thyroid Res. 2012;2012:351864. doi: 10.1155/2012/351864. Epub 2012 Dec 30. J Thyroid Res. 2012. PMID: 23365787 Free PMC article.
-
Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food.EFSA J. 2018 Nov 20;16(11):e05333. doi: 10.2903/j.efsa.2018.5333. eCollection 2018 Nov. EFSA J. 2018. PMID: 32625737 Free PMC article.
-
Endocrine disrupting chemicals: effects on pituitary, thyroid and adrenal glands.Endocrine. 2022 Dec;78(3):395-405. doi: 10.1007/s12020-022-03076-x. Epub 2022 May 23. Endocrine. 2022. PMID: 35604630 Free PMC article. Review.
References
-
- Arulmozhiraja S, Shiraishi F, Okumura T, Iida M, Takigami H, Edmonds JS, et al. Structural requirements for the interaction of 91 hydroxylated polychlorinated biphenyls with estrogen and thyroid hormone receptors. Toxicol Sci. 2005;84(1):49–62. - PubMed
-
- Bastomsky CH. Effects of a polychlorinated biphenyl mixture (Aroclor 1254) and DDT on biliary thyroxine excretion in rats. Endocrinology. 1974;95:1150–1155. - PubMed
-
- Bastomsky CH, Murthy PVN, Banovac K. Alterations in thyroxine metabolism produced by cutaneous application of microscope immersion oil: effects due to polychlorinated biphenyls. Endocrinology. 1976;98:1309–1314. - PubMed
-
- Bogazzi F, Raggi F, Ultimieri F, Russo D, Campomori A, McKinney JD, et al. Effects of a mixture of polychlorinated biphenyls (Aroclor 1254) on the transcriptional activity of thyroid hormone receptor. J Endocrinol Invest. 2003;26(10):972–978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

