Meeting report: hazard assessment for nanoparticles--report from an interdisciplinary workshop
- PMID: 18007999
- PMCID: PMC2072837
- DOI: 10.1289/ehp.10327
Meeting report: hazard assessment for nanoparticles--report from an interdisciplinary workshop
Abstract
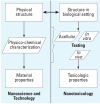
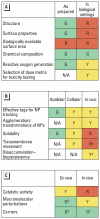
In this report we present the findings from a nanotoxicology workshop held 6-7 April 2006 at the Woodrow Wilson International Center for Scholars in Washington, DC. Over 2 days, 26 scientists from government, academia, industry, and nonprofit organizations addressed two specific questions: what information is needed to understand the human health impact of engineered nanoparticles and how is this information best obtained? To assess hazards of nanoparticles in the near-term, most participants noted the need to use existing in vivo toxicologic tests because of their greater familiarity and interpretability. For all types of toxicology tests, the best measures of nanoparticle dose need to be determined. Most participants agreed that a standard set of nanoparticles should be validated by laboratories worldwide and made available for benchmarking tests of other newly created nanoparticles. The group concluded that a battery of tests should be developed to uncover particularly hazardous properties. Given the large number of diverse materials, most participants favored a tiered approach. Over the long term, research aimed at developing a mechanistic understanding of the numerous characteristics that influence nanoparticle toxicity was deemed essential. Predicting the potential toxicity of emerging nanoparticles will require hypothesis-driven research that elucidates how physicochemical parameters influence toxic effects on biological systems. Research needs should be determined in the context of the current availability of testing methods for nanoscale particles. Finally, the group identified general policy and strategic opportunities to accelerate the development and implementation of testing protocols and ensure that the information generated is translated effectively for all stakeholders.
Keywords: nanomaterials; nanoparticle; nanotechnology; nanotoxicology; particle toxicology.
Figures

References
-
- Bucher J, Masten S, Moudgil B, Powers K, Roberts S, Walker N. Developing Experimental Approaches for the Evaluation of Toxicological Interactions of Nanoscale Materials. Final Workshop Report 3–4 November 2004, University of Florida; Gainesville, FL. Gainesville, FL: University of Florida; 2004. [[accessed 3 July 2007]]. pp. 1–37. Available: http://www.nanotoxicology.ufl.edu/workshop/images/NanoToxWorkshop.pdf.
-
- Chavanpatil MD, Khdair A, Panyam J. Nanoparticles for cellular drug delivery: mechanisms and factors influencing delivery. J Nanosci Nanotechnol. 2006;6(9–10):2651–2663. - PubMed
-
- Colvin VL. The potential environmental impact of engineered nanomaterials. Nat Biotechnol. 2003;21(10):1166–1170. Erratum in: Nat Biotechnol 2004 Jun 22, 6:760. - PubMed
-
- Gutierrez-Castillo ME, Roubicek DA, Cebrian-Garcia ME, De Vizcaya-Ruiz A, Sordo-Cedeno M, Ostrosky-Wegman P. Effect of chemical composition on the induction of DNA damage by urban airborne particulate matter. Environ Mol Mutagen. 2006;47(3):199–211. - PubMed
-
- Lynch I, Dawson KA, Linse S. Detecting cryptic epitopes created by nanoparticles. Sci STKE. 2006;2006(327):pe14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
