High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy
- PMID: 18019247
- PMCID: PMC2676886
- DOI: 10.1109/tuffc.2007.504
High speed imaging of bubble clouds generated in pulsed ultrasound cavitational therapy--histotripsy
Abstract
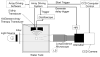
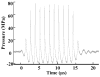
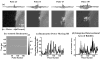
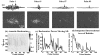
Our recent studies have demonstrated that mechanical fractionation of tissue structure with sharply demarcated boundaries can be achieved using short (< 20 micros), high intensity ultrasound pulses delivered at low duty cycles. We have called this technique histotripsy. Histotripsy has potential clinical applications where noninvasive tissue fractionation and/or tissue removal are desired. The primary mechanism of histotripsy is thought to be acoustic cavitation, which is supported by a temporally changing acoustic backscatter observed during the histotripsy process. In this paper, a fast-gated digital camera was used to image the hypothesized cavitating bubble cloud generated by histotripsy pulses. The bubble cloud was produced at a tissue-water interface and inside an optically transparent gelatin phantom which mimics bulk tissue. The imaging shows the following: (1) Initiation of a temporally changing acoustic backscatter was due to the formation of a bubble cloud; (2) The pressure threshold to generate a bubble cloud was lower at a tissue-fluid interface than inside bulk tissue; and (3) at higher pulse pressure, the bubble cloud lasted longer and grew larger. The results add further support to the hypothesis that the histotripsy process is due to a cavitating bubble cloud and may provide insight into the sharp boundaries of histotripsy lesions.
Figures





References
-
- Fry FJ, Kossoff G, Eggleton RC, Dunn F. Threshold ultrasound dosages for structural changes in the mammalian brain. J Acoust Soc Amer. 1970;48:1413–1417. - PubMed
-
- Dunn F, Fry FJ. Ultrasonic threshold dosages for the mammalian central nervous system. IEEE Trans Biomed Eng. 1971;18:253–256. - PubMed
-
- Frizzell LA, Lee CS, Aschenbach PD, Borrelli MJ, Morimoto RS, Dunn F. Involvement of ultrasonically induced cavitation in hind limb paralysis of the mouse neonate. J Acoust Soc Amer. 1983;74:1062–1065. - PubMed
-
- ter Haar GR, Daniels S, Morton K. Evidence for acoustic cavitation in vivo: Threshold for bubble formation with 0.75-MHz continuous-wave and pulsed beam. IEEE Trans Ultrason, Ferroelect, Freq Contr. 1986;33:162–164. - PubMed
-
- Hynynen K. Threshold for thermally significant cavitation in dog’s thigh muscle in vivo. Ultrasound Med Biol. 1991;17:157–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

