Connective tissue progenitor cell growth characteristics on textured substrates
- PMID: 18019838
- PMCID: PMC2676655
Connective tissue progenitor cell growth characteristics on textured substrates
Abstract
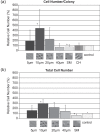
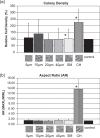
Growth characteristics of human connective tissue progenitor (CTP) cells were investigated on smooth and textured substrates, which were produced using MEMS (microelectromechanical systems) fabrication technology. Human bone marrow derived cells were cultured for 9 days under conditions promoting osteoblastic differentiation on polydimethylsiloxane (PDMS) substrates comprising smooth (non-patterned) surfaces (SMOOTH), 4 different cylindrical post micro-textures (POSTS) that were 7-10 microm high and 5, 10, 20, and 40 microm diameter, respectively, and channel micro-textures (CHANNELS) with curved cross-sections that were 11 microm high, 45 microm wide, and separated by 5 microm wide ridges. Standard glass-tissue culture surfaces were used as controls. Micro-textures resulted in the modification of CTP morphology, attachment, migration, and proliferation characteristics. Specifically, cells on POSTS exhibited more contoured morphology with closely packed cytoskeletal actin microfilaments compared to the more random orientation in cells grown on SMOOTH. CTP colonies on 10 gm-diameter POSTS exhibited higher cell number than any other POSTS, and a significant increase in cell number (442%) compared to colonies on SMOOTH (71%). On CHANNELS, colonies tended to be denser (229%) than on POSTS (up to 140% on 10 microm POSTS), and significantly more so compared to those on SMOOTH (104%).
Figures

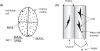













References
-
- Bone grafting CHC medical library and patient education 1999Bone grafts and bone substitutes Orthopedic Network News 1010–17.2003. Synthetic bone graft to be tested in revision hip surgery, Bioportfolio; (2005). Bone grafting, The Cleveland Clinic.
-
- Alaerts JA, De Cupere VM, et al. Surface characterization of poly(methyl methacrylate) microgrooved for contact guidance of mammalian cells. Biomaterials. 2001;22(12):1635–42. - PubMed
-
- Bauer TW, Muschler GF. Bone graft materials. An overview of the basic science. Clinical Orthopaedics and Related Research. 2000;371:10–27. - PubMed
-
- Brunette DM. Fibroblasts on micromachined substrata orient hierarchically to grooves of different dimensions. Experimental Cell Research. 1986;164(1):11–26. - PubMed
-
- Brunette DM. Spreading and orientation of epithelial cells on grooved substrata. Experimental Cell Research. 1986;167(1):203–17. - PubMed
