Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway
- PMID: 18021179
- PMCID: PMC6495898
- DOI: 10.1111/j.1365-2184.2007.00471.x
Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway
Abstract
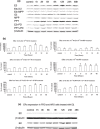
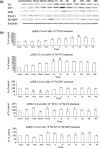
Objectives: Although thyroid cancer occurs much more frequently in females, the role of sex hormones in thyroid carcinogenesis is unknown. In this study, it has been investigated how 17beta-oestradiol (E2) influenced proliferation and growth of thyroid cancer cells.
Materials and methods: Cell proliferation and its related molecules were examined in thyroid papillary carcinoma cells (KAT5), follicular thyroid carcinoma cells (FRO) and anaplastic carcinoma cells (ARO). Levels of oestrogen receptor (ER) alpha and beta were regulated by their agonists (PPT and DPN), antagonists and siRNA.
Results: E2 promoted cell proliferation. Such an effect was positively related to ERalpha but negatively to ERbeta; PPT enhanced cell proliferation while DPN inhibited it. PPT increased Bcl-2 expression while DPN decreased it. DPN also elevated Bax expression. PPT elevated the level of phosphorylated extracellular signal-regulated kinase 1/2 (pERK1/2), suggesting a positive role of ERK1/2 in E2-induced cell proliferation. Knockdown of ERalpha significantly attenuated E2-mediated Bcl-2 and pERK1/2 expression. In contrast, knockdown of ERbeta markedly enhanced them.
Conclusions: Oestrogen stimulates proliferation of thyroid cancer cells, associated with increase in Bcl-2 and decrease in Bax levels in an ERK1/2-related pathway. Imbalance between ERalpha and ERbeta may contribute to thyroid carcinogenesis.
Figures







References
-
- Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M (2005) Survival versus apoptotic 17beta‐estradiol effect: role of ER alpha and ER beta activated non‐genomic signaling. J. Cell. Physiol. 203, 193–201. - PubMed
-
- Alexaki VI, Charalampopoulos I, Kampa M, Nifli AP, Hatzoglou A, Gravanis A, Castanas E (2006) Activation of membrane estrogen receptors induce pro‐survival kinases. J. Steroid Biochem. Mol. Biol. 98, 97–110. - PubMed
-
- Chen GG, Liu ZM, Vlantis AC, Tse GM, Leung BC, Van Hasselt CA (2004) Heme oxygenase‐1 protects against apoptosis induced by tumor necrosis factor‐alpha and cycloheximide in papillary thyroid carcinoma cells. J. Cell. Biochem. 92, 1246–1256. - PubMed
-
- Clarke RB (2003) Steroid receptors and proliferation in the human breast. Steroids 68, 789–794. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

