Human NCU-G1 can function as a transcription factor and as a nuclear receptor co-activator
- PMID: 18021396
- PMCID: PMC2233640
- DOI: 10.1186/1471-2199-8-106
Human NCU-G1 can function as a transcription factor and as a nuclear receptor co-activator
Abstract
Background: Novel, uncharacterised proteins represent a challenge in biochemistry and molecular biology. In this report we present an initial functional characterization of human kidney predominant protein, NCU-G1.
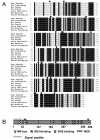
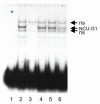
Results: NCU-G1 was found to be a highly conserved nuclear protein rich in proline with a molecular weight of approximately 44 kDa. It is localized on chromosome 1 and consists of 6 exons. Analysis of the amino acid sequence revealed no known transcription activation domains or DNA binding regions, however, four nuclear receptor boxes (LXXLL), and four SH3-interaction motives in addition to numerous potential phosphorylation sites were found. Two nuclear export signals were identified, but no nuclear localization signal. In man, NCU-G1 was found to be widely expressed at the mRNA level with especially high levels detected in prostate, liver and kidney. Electrophoretic mobility shift analysis showed specific binding of NCU-G1 to an oligonucleotide representing the footprint 1 element of the human cellular retinol-binding protein 1 gene promoter. NCU-G1 was found to activate transcription from this promoter and required presence of the footprint 1 element. In transiently transfected Drosophila Schneider S2 cells, we demonstrated that NCU-G1 functions as a co-activator for ligand-activated PPAR-alpha, resulting in an increased expression of a CAT reporter gene under control of the peroxisome proliferator-activated receptor-alpha responsive acyl-CoA oxidase promoter.
Conclusion: We propose that NCU-G1 is a dual-function protein capable of functioning as a transcription factor as well as a nuclear receptor co-activator.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

