Modulation of osteoblast gap junction connectivity by serum, TNFalpha, and TRAIL
- PMID: 18022159
- PMCID: PMC2714684
- DOI: 10.1016/j.yexcr.2007.10.010
Modulation of osteoblast gap junction connectivity by serum, TNFalpha, and TRAIL
Abstract
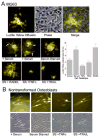
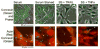
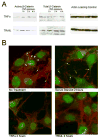
We studied the effects of serum growth factors and of TNF family proteins on osteoblast gap junction connectivity. Serum starvation of human MG63 osteosarcoma cells or nontransformed osteoblasts decreased connexin43 protein. TNFalpha or TRAIL reduced connexin43 further. Serum starvation redistributed gap junctions but did not reduce intercellular diffusion. In contrast, TNFalpha or TRAIL reduced gap junctions on cell processes and decreased intercellular diffusion. Effects of TNFs on connexin43 were mediated by lysosomal proteolysis. Activating analogs of cAMP increased connexin43 protein, but did not block effects of serum starvation, TNFalpha, or TRAIL on connexin43 protein. Connexin43 and connectivity recovered overnight if stimuli were withdrawn. Surprisingly, connexin43 mRNA increased in serum starvation and with TNFalpha or TRAIL. Since beta-catenin is a binding partner of connexin43, when connexin43 is degraded, beta-catenin activation may contribute to a reflexive increase in connexin43 transcription. We conclude that osteoblast connectivity is regulated by a multifactorial system that maintains intercellular connections. Serum starvation, TNFalpha and TRAIL augmented connexin43 degradation and connexin43 transcription. Cell-cell communication was maintained in serum starvation, which may model response to acute injury, but was sensitive to TNFs. These inflammatory agents mediated selective, reversible removal of connexin43 from cell processes.
Figures


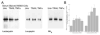
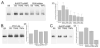
References
-
- Yellowley CE, Li Z, Zhou Z, Jacobs CR, Donahue HJ. Functional gap junctions between osteocytic and osteoblastic cells. J Bone Miner Res. 2000;15:209–217. - PubMed
-
- Cooper DM, Turinsky AL, Sensen CW, Hallgrimsson B. Quantitative 3D analysis of the canal network in cortical bone by micro-computed tomography. Anat Rec B New Anat. 2003;274:169–79. - PubMed
-
- Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. - PubMed
-
- Eberhardt AW, Yeager-Jones A, Blair HC. Regional trabecular bone matrix degeneration and osteocyte death in femora of glucocorticoid treated rabbits. Endocrinology. 2001;142:1333–1340. - PubMed
-
- Furlan F, Lecanda F, Screen J, Civitelli R. Proliferation, differentiation and apoptosis in connexin43-null osteoblasts. Cell Commun Adhes. 2001;8:367–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

