Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis
- PMID: 18022678
- PMCID: PMC2274006
- DOI: 10.1016/j.yfrne.2007.08.005
Estrogen receptors: their roles in regulation of vasopressin release for maintenance of fluid and electrolyte homeostasis
Abstract
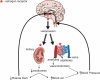
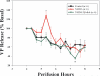
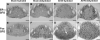
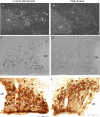
Long standing interest in the impact of gonadal steroid hormones on fluid and electrolyte balance has led to a body of literature filled with conflicting reports about gender differences, the effects of gonadectomy, hormone replacement, and reproductive cycles on plasma vasopressin (VP), VP secretion, and VP gene expression. This reflects the complexity of gonadal steroid hormone actions in the body resulting from multiple sites of action that impact fluid and electrolyte balance (e.g. VP target organs, afferent pathways regulating the VP neurons, and the VP secreting neurons themselves). It also reflects involvement of multiple types of estrogen receptors (ER) in these diverse sites including ERs that act as transcription factors regulating gene expression (i.e. the classic ERalpha as well as the more recently discovered ERbeta) and potentially G-protein coupled, membrane localized ERs that mediate rapid non-genomic actions of estrogen. Furthermore, altered expression of these receptors in physiologically diverse conditions of fluid and electrolyte balance contributes to the difficulty of using simplistic approaches such as gender comparisons, gonadectomy, and hormone replacement to assess the role of gonadal steroids in regulation of VP secretion for maintenance of fluid and electrolyte homeostasis. This review catalogs these inconsistencies and provides a frame work for understanding them by describing: (1) the effect of gonadal steroids on target organ responsiveness to VP; (2) the expression of multiple types of estrogen receptors in the VP neurons and in brain regions monitoring feedback signals from the periphery; and (3) the impact of dehydration and hyponatremia on expression of these receptors.
Figures

References
-
- Altura BM. Sex and estrogens and responsiveness of terminal arterioles to neurohypophyseal hormones and catecholamines. J Pharmacol Exp Ther. 1975;193(2):403–412. - PubMed
-
- Altura BM, Altura BT. Vascular smooth muscle and neurohypophyseal hormones. Federation Proceedings. 1977;36:1853–1860. - PubMed
-
- Berghorn KA, Knapp LT, Hoffman GE, Sherman TG. Induction of glucocorticoid receptor expression in hypothalamic magnocellular vasopressin neurons during chronic hypoosmolality. Endocrinology. 1995;136:804–811. - PubMed
-
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. - PubMed
-
- Burbach JPH, DeHoop MJ, Schmale H, Richter D, DeKloet ER, TenHaaf JA, DeWied D. Differential responses to osmotic stress of vasopressin-neurophysin mRNA in hypothalamic nuclei. Neuroendocrinology. 1984;39:582–584. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

